Considerations in the Preclinical Assessment of the Safety of Antisense Oligonucleotides
- PMID: 36579950
- PMCID: PMC9940817
- DOI: 10.1089/nat.2022.0061
Considerations in the Preclinical Assessment of the Safety of Antisense Oligonucleotides
Abstract
The nucleic acid therapeutics field has made tremendous progress in the past decades. Continuous advances in chemistry and design have led to many successful clinical applications, eliciting even more interest from researchers including both academic groups and drug development companies. Many preclinical studies in the field focus on improving the delivery of antisense oligonucleotide drugs (ONDs) and/or assessing their efficacy in target tissues, often neglecting the evaluation of toxicity, at least in early phases of development. A series of consensus recommendations regarding regulatory considerations and expectations have been generated by the Oligonucleotide Safety Working Group and the Japanese Research Working Group for the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use S6 and Related Issues (WGS6) in several white papers. However, safety aspects should also be kept in sight in earlier phases while screening and designing OND to avoid subsequent failure in the development phase. Experts and members of the network "DARTER," a COST Action funded by the Cooperation in Science and Technology of the EU, have utilized their collective experience working with OND, as well as their insights into OND-mediated toxicities, to generate a series of consensus recommendations to assess OND toxicity in early stages of preclinical research. In the past few years, several publications have described predictive assays, which can be used to assess OND-mediated toxicity in vitro or ex vivo to filter out potential toxic candidates before moving to in vivo phases of preclinical development, that is, animal toxicity studies. These assays also have the potential to provide translational insight since they allow a safety evaluation in human in vitro systems. Yet, small preliminary in vivo studies should also be considered to complement this early assessment. In this study, we summarize the state of the art and provide guidelines and recommendations on the different tests available for these early stage preclinical assessments.
Keywords: antisense oligonucleotides; early preclinical safety assessment; predictive assays; toxicity.
Conflict of interest statement
None of this funding represents a conflict of interest with the content of this review.
Figures
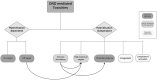
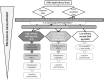
References
-
- Maquat LE. (2004). Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol 5:89–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
