Accessing Chiral Pyrrolodiketopiperazines under Organocatalytic Conditions
- PMID: 36579971
- PMCID: PMC10018776
- DOI: 10.1021/acs.orglett.2c03924
Accessing Chiral Pyrrolodiketopiperazines under Organocatalytic Conditions
Abstract
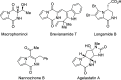
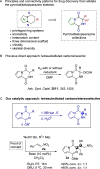
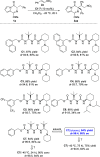
The production of chiral pyrrolodiketopiperazines under organocatalytic conditions demonstrates the capacity of bicyclic acylpyrrol lactims to perform as pronucleophiles in direct carbon-carbon bond forming reactions. The good performance of ureidoaminal-derived Brønsted bases in the Michael addition to nitroolefins affords these heterocyclic scaffolds with high skeleton diversity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Shiokawa Z.; Kashiwabara E.; Yoshidome D.; Fukase K.; Inuki S.; Fujimoto Y. Discovery of a Novel Scaffold as an Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Based on the Pyrrolopiperazinone Alkaloid, Longamide B. ChemMedChem. 2016, 11, 2682–2689. 10.1002/cmdc.201600446. - DOI - PubMed
- Jansen R.; Sood S.; Mohr K. I.; Kunze B.; Irschik H.; Stadler M.; Müller R. Nannozinones and Sorazinones, Unprecedented Pyrazinones from Myxobacteria. J. Nat. Prod. 2014, 77, 2545–2552. 10.1021/np500632c. - DOI - PubMed
- Song F. H.; Liu X. R.; Guo H.; Ren B.; Chen C. X.; Piggott A. M.; Yu K.; Gao H.; Wang Q.; Liu M.; Liu X. T.; Dai H. Q.; Zhang L. X.; Capon R. J. Brevianamides with Antitubercular Potential from a Marine-Derived Isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. 10.1021/ol302051x. - DOI - PubMed
- Miyashiro J.; Woods K. W.; Park C. H.; Liu X.; Shi Y.; Johnson E. F.; Bouska J. J.; Olson A. M.; Luo Y.; Fry E. H.; Giranda V. L.; Penning T. D. Synthesis and SAR of novel tricyclic quinoxalinone inhibitors of poly(ADPribose) polymerase-1 (PARP-1). Bioorg. Med. Chem. Lett. 2009, 19, 4050–4054. 10.1016/j.bmcl.2009.06.016. - DOI - PubMed
- Rowan D. D.; Hunt M. B.; Gaynor D. L. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte acremonium-ioliae. J. Chem. Soc., Chem. Commun. 1986, 935–936. 10.1039/c39860000935. - DOI - PubMed
-
- Trigos A. Macrophominol, a diketopiperazine from cultures of Macrophomina phaseolina. Phytochemistry 1995, 40, 1697–1698. 10.1016/0031-9422(95)00626-I. - DOI
- Cafieri F.; Fattorusso E.; Taglialatela-Scafati O. Novel Bromopyrrole Alkaloids from the Sponge Agelas dispar. J. Nat. Prod. 1998, 61, 122–125. 10.1021/np970323h. - DOI - PubMed
-
See ref (1b)
-
-
See, for instance:
- Huigens R. W. III; Morrison K. C.; Hickling R. W.; Flood T. A. Jr.; Richter M. F.; Hergenrother P. J. A ring distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nat. Chem. 2013, 5, 195–202. 10.1038/nchem.1549. - DOI - PMC - PubMed
- Rafferty R. J.; Hicklin R. W.; Maloof K. A.; Hergenrother P. J. Synthesis of complex and diverse compounds through ring distortion of abietic acid. Angew. Chem., Int. Ed. 2014, 53, 220–224. 10.1002/anie.201308743. - DOI - PubMed
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

