Serotype distribution of Streptococcus pneumoniae and pneumococcal vaccine coverage in adults in Turkey between 2015 and 2018
- PMID: 36579976
- PMCID: PMC9809394
- DOI: 10.1080/07853890.2022.2160877
Serotype distribution of Streptococcus pneumoniae and pneumococcal vaccine coverage in adults in Turkey between 2015 and 2018
Abstract
Objective: To evaluate the serotype distribution and antibiotic resistance in pneumococcal infections in adults and to provide a perspective regarding serotype coverage of both current and future pneumococcal vaccines.
Patients and methods: This passive surveillance study was conducted with the Streptococcus pneumoniae strains isolated from the specimens of patients with pneumonia (materials isolated from bronchoalveolar lavage), bacteraemia, meningitis, pleuritis and peritonitis between 2015 and 2018. Serogrouping and serotyping were performed by latex particle agglutination and by conventional Quellung reaction using commercial type-specific antisera, respectively. The strains were analysed for penicillin, cefotaxime, erythromycin and moxifloxacin susceptibilities by E-test.
Results: In the whole study group (410 samples from adults aged ≥18 years), the most frequent serotypes were 3 (14.1%), 19 F (12%) and 1 (9.3%). The vaccine coverage for PCV13, PCV15, PCV20 and PPV23 was 63.9%, 66.6%, 74.1% and 75.9%, respectively, in all isolates. Penicillin non-susceptibility in invasive pneumococcal disease (IPD) was 70.8% and 57.1% in the patients aged <65 and ≥65 years, respectively. About 21.1% and 4.3% of the patients with and without IPD had cefotaxime resistance. Non-susceptibility to erythromycin and moxifloxacin was 38.2% and 1.2%, respectively.
Conclusions: The results revealed that novel PCV vaccines may provide improved coverage as compared with the currently available vaccine, PCV13. The significant antibiotic resistance rates imply the need to extend the serotype coverage of the vaccines. Continuing the surveillance in pneumococcal diseases is critical to explore the serotype distribution and incidence changes of IPD cases in the population and to inform policy makers to make necessary improvements in the national immunization programmes.Key messagesThis multicentre study demonstrated the most recent serotype distribution and antibiotic resistance in adult population in Turkey.Shifting from PCV13 to novel conjugated vaccines will significantly increase the coverage.Continuing the surveillance in pneumococcal diseases is critical to explore the serotype distribution changes and the incidence of cases with invasive pneumococcal disease in the population.
Keywords: Streptococcus pneumoniae; antibiotic susceptibility; serotype; surveillance; vaccine coverage.
Conflict of interest statement
Mehmet Ceyhan received consulting fees and research funding from Pfizer Pharmaceuticals, Istanbul, Turkey and all the remaining authors report no conflict of interest.
Figures

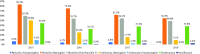

References
-
- Drijkoningen JJ, Rohde GG.. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20:45–51. - PubMed
-
- O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. - PubMed
-
- Curcio D, Cané A, Isturiz R.. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–35. - PubMed
-
- Senol E, Azap A, Erbay A, et al. Pneumococcal vaccine as one of the immunization coverage targets for adulthood vaccines: a consensus report of the study group for adult immunization of the Turkish Society of Clinical Microbiology and Infectious Diseases. Klimik Dergisi. 2018;31(1):2–18. Turkish.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
