Dissecting the complexity of pediatric blood transfusions and risk of adverse reactions in Aotearoa New Zealand
- PMID: 36580030
- PMCID: PMC10497383
- DOI: 10.2450/2022.0178-22
Dissecting the complexity of pediatric blood transfusions and risk of adverse reactions in Aotearoa New Zealand
Abstract
Background: Children have different clinical and physiological drivers for transfusion from adult recipients. However, adverse transfusion reactions (ATRs) in pediatric patients are usually reported using the same criteria as for adults. Broad assessments of pediatric ATRs neglect substantial variation in different developmental stages.
Materials and methods: This retrospective study included 342,950 patients, ~2.43 million transfusions, and 5,540 ATR reports collated from New Zealand hospitals between 2005 and 2021. Using 16 years as the upper age limit, 138,856 pediatric transfusions and 402 pediatric ATR reports were identified and dissected at three levels: pediatric as a whole, pediatric developmental stage (i.e., neonate, infant, preschool, and school), and chronological age to identify patients at high risk of ATRs. Multivariate logistic regression analysis was followed to quantify risk factors.
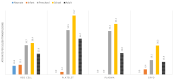
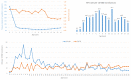
Results: Pediatric recipients had a higher ATR risk than adults (p=6.9-07) but the high risk was associated mainly with children older than 2 years. Neonates and infants accounted for 75.0% of pediatric recipients but had much lower ATR rates than adults. Pediatric transfusion recipients showed a clear male bias prior to age 11 years and then a female bias. However, gender difference in experiencing ATRs was significant only after age 13 years (p=2.3-04). Analyses focusing on the high-risk group revealed allergic reactions being the cause of the elevated risk and identified the main risk factors of number of transfusions (p=4.5-10) and multiple types of components transfused (p=2.0-13).
Discussion: The identified ATR risk factors signal linkage with the biological drivers for transfusion. Low ATR rates in infancy could also be attributed to use of neonatal components, low transfusions per patient, and less developed immunity. The relative increase in female recipients from age 11 may be associated with increased red blood cell demand following puberty.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures


Similar articles
-
Pediatric blood transfusions in Colombia: Dissecting adverse reaction trends and age dynamics.Transfusion. 2025 Jan;65(1):100-109. doi: 10.1111/trf.18074. Epub 2024 Nov 24. Transfusion. 2025. PMID: 39580794
-
Contribution of donor- and recipient-associated factors to allergic transfusion reactions to platelets.Transfusion. 2021 Mar;61(3):744-753. doi: 10.1111/trf.16221. Epub 2020 Dec 13. Transfusion. 2021. PMID: 33314235
-
A retrospective observational study to assess adverse transfusion reactions of patients with and without prior transfusion history.Vox Sang. 2015 Apr;108(3):243-50. doi: 10.1111/vox.12208. Epub 2014 Dec 23. Vox Sang. 2015. PMID: 25536173
-
Preventing adverse reactions in pediatric transfusions using washed platelet concentrate.Pediatr Int. 2021 Apr;63(4):391-403. doi: 10.1111/ped.14572. Epub 2021 Mar 27. Pediatr Int. 2021. PMID: 33290634 Review.
-
Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions.Transfusion. 2002 Jan;42(1):37-43. doi: 10.1046/j.1537-2995.2002.00007.x. Transfusion. 2002. PMID: 11896310 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
