Association of COVID-19 Vaccination With Breakthrough Infections and Complications in Patients With Cancer
- PMID: 36580318
- PMCID: PMC10020872
- DOI: 10.1001/jamaoncol.2022.6815
Association of COVID-19 Vaccination With Breakthrough Infections and Complications in Patients With Cancer
Abstract
Importance: Patients with cancer are known to have increased risk of COVID-19 complications, including death.
Objective: To determine the association of COVID-19 vaccination with breakthrough infections and complications in patients with cancer compared to noncancer controls.
Design, setting, and participants: Retrospective population-based cohort study using linked administrative databases in Ontario, Canada, in residents 18 years and older who received COVID-19 vaccination. Three matched groups were identified (based on age, sex, type of vaccine, date of vaccine): 1:4 match for patients with hematologic and solid cancer to noncancer controls (hematologic and solid cancers separately analyzed), 1:1 match between patients with hematologic and patients with solid cancer.
Exposures: Cancer diagnosis.
Main outcomes and measures: Outcomes occurring 14 days after receipt of second COVID-19 vaccination dose: primary outcome was SARS-CoV-2 breakthrough infection; secondary outcomes were emergency department visit, hospitalization, and death within 4 weeks of SARS-CoV-2 infection (end of follow-up March 31, 2022). Multivariable cumulative incidence function models were used to obtain adjusted hazard ratio (aHR) and 95% CIs.
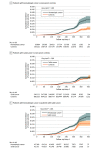
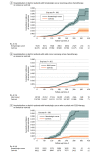
Results: A total of 289 400 vaccinated patients with cancer (39 880 hematologic; 249 520 solid) with 1 157 600 matched noncancer controls were identified; the cohort was 65.4% female, and mean (SD) age was 66 (14.0) years. SARS-CoV-2 breakthrough infection was higher in patients with hematologic cancer (aHR, 1.33; 95% CI, 1.20-1.46; P < .001) but not in patients with solid cancer (aHR, 1.00; 95% CI, 0.96-1.05; P = .87). COVID-19 severe outcomes (composite of hospitalization and death) were significantly higher in patients with cancer compared to patients without cancer (aHR, 1.52; 95% CI, 1.42-1.63; P < .001). Risk of severe outcomes was higher among patients with hematologic cancer (aHR, 2.51; 95% CI, 2.21-2.85; P < .001) than patients with solid cancer (aHR, 1.43; 95% CI, 1.24-1.64; P < .001). Patients receiving active treatment had a further heightened risk for COVID-19 severe outcomes, particularly those who received anti-CD20 therapy. Third vaccination dose was associated with lower infection and COVID-19 complications, except for patients receiving anti-CD20 therapy.
Conclusions and relevance: In this large population-based cohort study, patients with cancer had greater risk of SARS-CoV-2 infection and worse outcomes than patients without cancer, and the risk was highest for patients with hematologic cancer and any patients with cancer receiving active treatment. Triple vaccination was associated with lower risk of poor outcomes.
Conflict of interest statement
Figures
Comment in
-
Refusal of vaccination against influenza and COVID-19 in patients with solid cancers: from bio-ethical issues to solutions.Eur J Cancer. 2023 Apr;183:139-141. doi: 10.1016/j.ejca.2023.01.028. Epub 2023 Feb 9. Eur J Cancer. 2023. PMID: 36857818 Free PMC article. No abstract available.
-
Severe COVID-19 outcomes and hematological cancers: the clot thickens-are platelets the culprit?Transl Cancer Res. 2023 Sep 30;12(9):2425-2428. doi: 10.21037/tcr-23-672. Epub 2023 Aug 11. Transl Cancer Res. 2023. PMID: 37859735 Free PMC article. No abstract available.
References
-
- Lee LYW, Starkey T, Ionescu MC, et al. ; NCRI Consumer Forum . Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23(6):748-757. doi:10.1016/S1470-2045(22)00202-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

