Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons - 29 U.S. Jurisdictions, May 22-September 3, 2022
- PMID: 36580416
- PMCID: PMC9812445
- DOI: 10.15585/mmwr.mm715152a2
Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons - 29 U.S. Jurisdictions, May 22-September 3, 2022
Abstract
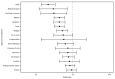
As of November 14, 2022, monkeypox (mpox) cases had been reported from more than 110 countries, including 29,133 cases in the United States.* Among U.S. cases to date, 95% have occurred among males (1). After the first confirmed U.S. mpox case on May 17, 2022, limited supplies of JYNNEOS vaccine (Modified Vaccinia Ankara vaccine, Bavarian Nordic) were made available to jurisdictions for persons exposed to mpox. JYNNEOS vaccine was approved by the Food and Drug Administration (FDA) in 2019 as a 2-dose series (0.5 mL per dose, administered subcutaneously) to prevent smallpox and mpox disease.† On August 9, 2022, FDA issued an emergency use authorization to allow administration of JYNNEOS vaccine by intradermal injection (0.1 mL per dose) (2). A previous report on U.S. mpox cases during July 31-September 3, 2022, suggested that 1 dose of vaccine offers some protection against mpox (3). This report describes demographic and clinical characteristics of cases occurring ≥14 days after receipt of 1 dose of JYNNEOS vaccine and compares them with characteristics of cases among unvaccinated persons with mpox and with the vaccine-eligible vaccinated population in participating jurisdictions. During May 22-September 3, 2022, among 14,504 mpox cases reported from 29 participating U.S. jurisdictions,§ 6,605 (45.5%) had available vaccination information and were included in the analysis. Among included cases, 276 (4.2%) were among persons who had received 1 dose of vaccine ≥14 days before illness onset. Mpox cases that occurred in these vaccinated persons were associated with lower percentage of hospitalization (2.1% versus 7.5%), fever, headache, malaise, myalgia, and chills, compared with cases in unvaccinated persons. Although 1 dose of JYNNEOS vaccine offers some protection from disease, mpox infection can occur after receipt of 1 dose, and the duration of protection conferred by 1 dose is unknown. Providers and public health officials should therefore encourage persons at risk for acquiring mpox to complete the 2-dose vaccination series and provide guidance and education regarding nonvaccine-related prevention strategies (4).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Emma Creegan reports receiving an honorarium for a fall 2021 speaking event at Brown University. No other potential conflicts of interest were disclosed.
Figures
References
-
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of JYNNEOS (smallpox and monkeypox vaccine, live, non-replicating) for prevention of monkeypox disease in individuals determined to be at high risk for monkeypox infection. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/160774/download#:~:text=The%20FDA%20has%20gran...
-
- CDC. Mpox vaccination basics. Atlanta, GA: US Department of Health and Human Services; CDC; 2022. Accessed December 7, 2022. https://www.cdc.gov/poxvirus/monkeypox/vaccines/vaccine-basics.html
-
- CDC. Technical report 3: multi-national monkeypox outbreak, United States, 2022. Atlanta, GA: US Department of Health and Human Services; CDC; 2022. Accessed October 26, 2022. https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/repor...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

