Associations of genetically predicted fatty acid levels across the phenome: A mendelian randomisation study
- PMID: 36580444
- PMCID: PMC9799317
- DOI: 10.1371/journal.pmed.1004141
Associations of genetically predicted fatty acid levels across the phenome: A mendelian randomisation study
Abstract
Background: Fatty acids are important dietary factors that have been extensively studied for their implication in health and disease. Evidence from epidemiological studies and randomised controlled trials on their role in cardiovascular, inflammatory, and other diseases remains inconsistent. The objective of this study was to assess whether genetically predicted fatty acid concentrations affect the risk of disease across a wide variety of clinical health outcomes.
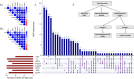
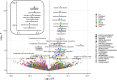
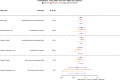
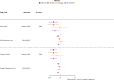
Methods and findings: The UK Biobank (UKB) is a large study involving over 500,000 participants aged 40 to 69 years at recruitment from 2006 to 2010. We used summary-level data for 117,143 UKB samples (base dataset), to extract genetic associations of fatty acids, and individual-level data for 322,232 UKB participants (target dataset) to conduct our discovery analysis. We studied potentially causal relationships of circulating fatty acids with 845 clinical diagnoses, using mendelian randomisation (MR) approach, within a phenome-wide association study (PheWAS) framework. Regression models in PheWAS were adjusted for sex, age, and the first 10 genetic principal components. External summary statistics were used for replication. When several fatty acids were associated with a health outcome, multivariable MR and MR-Bayesian method averaging (MR-BMA) was applied to disentangle their causal role. Genetic predisposition to higher docosahexaenoic acid (DHA) was associated with cholelithiasis and cholecystitis (odds ratio per mmol/L: 0.76, 95% confidence interval: 0.66 to 0.87). This was supported in replication analysis (FinnGen study) and by the genetically predicted omega-3 fatty acids analyses. Genetically predicted linoleic acid (LA), omega-6, polyunsaturated fatty acids (PUFAs), and total fatty acids (total FAs) showed positive associations with cardiovascular outcomes with support from replication analysis. Finally, higher genetically predicted levels of DHA (0.83, 0.73 to 0.95) and omega-3 (0.83, 0.75 to 0.92) were found to have a protective effect on obesity, which was supported using body mass index (BMI) in the GIANT consortium as replication analysis. Multivariable MR analysis suggested a direct detrimental effect of LA (1.64, 1.07 to 2.50) and omega-6 fatty acids (1.81, 1.06 to 3.09) on coronary heart disease (CHD). MR-BMA prioritised LA and omega-6 fatty acids as the top risk factors for CHD. Although we present a range of sensitivity analyses to the address MR assumptions, horizontal pleiotropy may still bias the reported associations and further evaluation in clinical trials is needed.
Conclusions: Our study suggests potentially protective effects of circulating DHA and omega-3 concentrations on cholelithiasis and cholecystitis and on obesity, highlighting the need to further assess them as prevention treatments in clinical trials. Moreover, our findings do not support the supplementation of unsaturated fatty acids for cardiovascular disease prevention.
Copyright: © 2022 Zagkos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DG is employed part-time by Novo Nordisk. VZ is a paid statistical consultant on PLOS Medicine’s statistical board.
Figures


References
-
- Nagy K, Tiuca I. Importance of Fatty Acids in Physiopathology of Human Body. 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

