Loss of Slc12a2 specifically in pancreatic β-cells drives metabolic syndrome in mice
- PMID: 36580474
- PMCID: PMC9799326
- DOI: 10.1371/journal.pone.0279560
Loss of Slc12a2 specifically in pancreatic β-cells drives metabolic syndrome in mice
Abstract
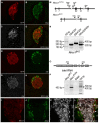
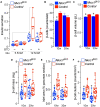
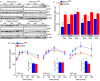
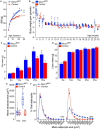
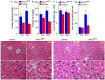
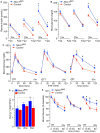
The risk of type-2 diabetes and cardiovascular disease is higher in subjects with metabolic syndrome, a cluster of clinical conditions characterized by obesity, impaired glucose metabolism, hyperinsulinemia, hyperlipidemia and hypertension. Diuretics are frequently used to treat hypertension in these patients, however, their use has long been associated with poor metabolic outcomes which cannot be fully explained by their diuretic effects. Here, we show that mice lacking the diuretic-sensitive Na+K+2Cl-cotransporter-1 Nkcc1 (Slc12a2) in insulin-secreting β-cells of the pancreatic islet (Nkcc1βKO) have reduced in vitro insulin responses to glucose. This is associated with islet hypoplasia at the expense of fewer and smaller β-cells. Remarkably, Nkcc1βKO mice excessively gain weight and progressive metabolic syndrome when fed a standard chow diet ad libitum. This is characterized by impaired hepatic insulin receptor activation and altered lipid metabolism. Indeed, overweight Nkcc1βKO but not lean mice had fasting and fed hyperglycemia, hypertriglyceridemia and non-alcoholic steatohepatitis. Notably, fasting hyperinsulinemia was detected earlier than hyperglycemia, insulin resistance, glucose intolerance and increased hepatic de novo gluconeogenesis. Therefore, our data provide evidence supporting the novel hypothesis that primary β-cell defects related to Nkcc1-regulated intracellular Cl-homeostasis and β-cell growth can result in the development of metabolic syndrome shedding light into additional potential mechanisms whereby chronic diuretic use may have adverse effects on metabolic homeostasis in susceptible individuals.
Copyright: © 2022 Abdelgawad et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109(1):42–6. doi: 10.1161/01.CIR.0000108926.04022.0C . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

