DNA polymerase θ protects leukemia cells from metabolically induced DNA damage
- PMID: 36580665
- PMCID: PMC10273171
- DOI: 10.1182/blood.2022018428
DNA polymerase θ protects leukemia cells from metabolically induced DNA damage
Abstract
Leukemia cells accumulate DNA damage, but altered DNA repair mechanisms protect them from apoptosis. We showed here that formaldehyde generated by serine/1-carbon cycle metabolism contributed to the accumulation of toxic DNA-protein crosslinks (DPCs) in leukemia cells, especially in driver clones harboring oncogenic tyrosine kinases (OTKs: FLT3(internal tandem duplication [ITD]), JAK2(V617F), BCR-ABL1). To counteract this effect, OTKs enhanced the expression of DNA polymerase theta (POLθ) via ERK1/2 serine/threonine kinase-dependent inhibition of c-CBL E3 ligase-mediated ubiquitination of POLθ and its proteasomal degradation. Overexpression of POLθ in OTK-positive cells resulted in the efficient repair of DPC-containing DNA double-strand breaks by POLθ-mediated end-joining. The transforming activities of OTKs and other leukemia-inducing oncogenes, especially of those causing the inhibition of BRCA1/2-mediated homologous recombination with and without concomitant inhibition of DNA-PK-dependent nonhomologous end-joining, was abrogated in Polq-/- murine bone marrow cells. Genetic and pharmacological targeting of POLθ polymerase and helicase activities revealed that both activities are promising targets in leukemia cells. Moreover, OTK inhibitors or DPC-inducing drug etoposide enhanced the antileukemia effect of POLθ inhibitor in vitro and in vivo. In conclusion, we demonstrated that POLθ plays an essential role in protecting leukemia cells from metabolically induced toxic DNA lesions triggered by formaldehyde, and it can be targeted to achieve a therapeutic effect.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: H.F.F. serves on the advisory board of Jazz Pharmaceuticals and Incyte. R.T.P. is a co-founder and chief scientific officer of Recombination Therapeutics, LLC. M.S.T. received research funding from AbbVie, Orsenix, BioSight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; is on advisory boards for AbbVie, Daiichi-Sankyo, Orsenix, KAHR, Jazz Pharma, Roche, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, Ipsen Biopharmaceuticals, and Cellularity; and received royalties from UpToDate, the Data and Safety Monitoring Board (DSMB) HOVON protocol Ho156, and the Adjudication Committee Foghorn Therapeutics protocol FHD-286. R.L.L. is on the supervisory board of Qiagen; is a scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis; receives research support from Ajax, Zentalis, and Abbvie; has consulted for Incyte, Janssen, and Astra Zeneca; and has received honoraria from Astra Zeneca for invited lectures. The remaining authors declare no competing financial interests.
Figures



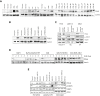





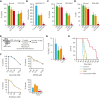
Comment in
-
Targeting leukemia with a metabolic genotoxin.Blood. 2023 May 11;141(19):2293-2295. doi: 10.1182/blood.2022019509. Blood. 2023. PMID: 37166927 No abstract available.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Nagy K, Pollreisz F, Takáts Z, Vékey K. Atmospheric pressure chemical ionization mass spectrometry of aldehydes in biological matrices. Rapid Commun Mass Spectrom. 2004;18(20):2473–2478. - PubMed
-
- Luo W, Li H, Zhang Y, Ang CY. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 2001;753:253–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UG1 CA233290/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- R01 CA247707/CA/NCI NIH HHS/United States
- P01 CA247773/CA/NCI NIH HHS/United States
- R01 CA244044/CA/NCI NIH HHS/United States
- R01 CA237286/CA/NCI NIH HHS/United States
- R01 CA237165/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- R01 CA186238/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- R01 CA244179/CA/NCI NIH HHS/United States
- R01 CA227830/CA/NCI NIH HHS/United States
- UG1 CA233332/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

