Regulation and function of the mammalian tricarboxylic acid cycle
- PMID: 36581208
- PMCID: PMC9871338
- DOI: 10.1016/j.jbc.2022.102838
Regulation and function of the mammalian tricarboxylic acid cycle
Abstract
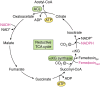
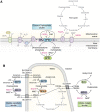
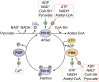
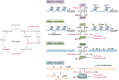
The tricarboxylic acid (TCA) cycle, otherwise known as the Krebs cycle, is a central metabolic pathway that performs the essential function of oxidizing nutrients to support cellular bioenergetics. More recently, it has become evident that TCA cycle behavior is dynamic, and products of the TCA cycle can be co-opted in cancer and other pathologic states. In this review, we revisit the TCA cycle, including its potential origins and the history of its discovery. We provide a detailed accounting of the requirements for sustained TCA cycle function and the critical regulatory nodes that can stimulate or constrain TCA cycle activity. We also discuss recent advances in our understanding of the flexibility of TCA cycle wiring and the increasingly appreciated heterogeneity in TCA cycle activity exhibited by mammalian cells. Deeper insight into how the TCA cycle can be differentially regulated and, consequently, configured in different contexts will shed light on how this pathway is primed to meet the requirements of distinct mammalian cell states.
Keywords: Krebs cycle; bioenergetics; cell metabolism; citric acid cycle; tricarboxylic acid cycle.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest P. K. A. and L. W. S. F. are authors on patent applications that cover links between cellular metabolism and cell fate control.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

