SH003 Causes ER Stress-mediated Apoptosis of Breast Cancer Cells via Intracellular ROS Production
- PMID: 36581346
- PMCID: PMC9806670
- DOI: 10.21873/cgp.20367
SH003 Causes ER Stress-mediated Apoptosis of Breast Cancer Cells via Intracellular ROS Production
Abstract
Background/aim: Breast cancer is one of the most common cancers in women all over the world and new treatment options are urgent. ER stress in cancer cells results in apoptotic cell death, and it is being proposed as a new therapeutic target. SH003, a newly developed herbal medicine, has been reported to have anti-cancer effects. However, its molecular mechanism is not yet clearly defined.
Materials and methods: Microarray was performed to check the differential gene expression patterns in various breast cancer cell lines. Cell viability was measured by MTT assays to detect cytotoxic effects. Annexin V-FITC and 7AAD staining, TUNEL assay and DCF-DA staining were analyzed by flow cytometry to evaluate apoptosis and ROS levels, respectively. Protein expression was examined in SH003-breast cancer cells using immunoblotting assays. The expression of C/EBP Homologous Protein (CHOP) mRNA was measured by real-time PCR. The effects of CHOP by SH003 treatment were investigated using transfection method.
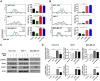
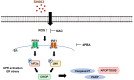
Results: Herein, we investigated the molecular mechanisms through which SH003 causes apoptosis of human breast cancer cells. Both cell viability and apoptosis assays confirmed the SH003-induced apoptosis of breast cancer cells. Meanwhile, SH003 altered the expression patterns of several genes in a variety of breast cancer cell lines. More specifically, it upregulated gene sets including the response to unfolded proteins, independently of the breast cancer cell subtype. In addition, SH003-induced apoptosis was due to an increase in ROS production and an activation of the ER stress-signaling pathway. Moreover, CHOP gene silencing blocked SH003-induced apoptosis.
Conclusion: SH003 causes apoptosis of breast cancer cells by upregulating ROS production and activating the ER stress-mediated pathway. Thus, our findings suggest that SH003 can be a potential therapeutic agent for breast cancer.
Keywords: Breast cancer; CHOP; ER stress; ROS; SH003.
Copyright © 2023, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
