Epidemiology of Developmental and Epileptic Encephalopathy and of Intellectual Disability and Epilepsy in Children
- PMID: 36581463
- PMCID: PMC10065214
- DOI: 10.1212/WNL.0000000000206758
Epidemiology of Developmental and Epileptic Encephalopathy and of Intellectual Disability and Epilepsy in Children
Abstract
Background and objectives: We aimed to determine the population-based cumulative incidence and prevalence of developmental and epileptic encephalopathies (DEEs) and intellectual disability and epilepsy (ID+E) in children. We analyzed the cumulative incidence of specific epilepsy syndromes.
Methods: Children younger than 16 years with a DEE or ID+E were ascertained using EEG records from 2000 to 2016 in the Wellington region of New Zealand. Epilepsy syndromes were diagnosed on medical record and EEG review. Point prevalence and cumulative incidence for children with epilepsy and developmental impairment, DEE and ID+E were calculated. Cumulative incidence for each epilepsy syndrome was calculated.
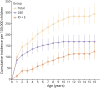
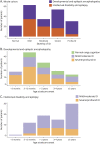
Results: The cohort comprised 235 children (58% male) with developmental impairment and epilepsy, including 152 (65%) with DEE and 83 (35%) with ID+E. The median age of seizure onset was 15.4 months (range day 1-15 years). The median follow-up from seizure onset was 7.9 years (range 0-18.2 years). Point prevalence for the broad group of children with epilepsy and developmental impairment was 175/100,000 children (95% CI 149-203; DEE 112 and ID+E 63/100,000 children). Cumulative incidence for DEE was 169/100,000 children (95% CI 144-199) and that for ID+E was 125/100,000 children (95% CI 95.4-165). Cumulative incidence per 100,000 children was as follows: infantile epileptic spasms syndrome 58.2 (95% CI 45.0-75.3), epilepsy with myoclonic-atonic seizures 16.4 (95% CI 9.69-27.7), Lennox-Gastaut syndrome 13.2 (95% CI 4.1-41.9), and Dravet syndrome 5.1 (95% CI 2.1-12.2). Fifty/152 (33%) of children with DEE and 70/83 (84%) with ID+E could not be diagnosed with a known epilepsy syndrome.
Discussion: Epilepsy and developmental impairment before the age of 16 years occurs in 1 in 340 children, with 1 in 590 having a DEE and 1 in 800 having ID+E. These individuals require significant health and community resources; therefore, these data will inform complex health service and education planning. Epidemiologic studies have focused on early childhood-onset DEEs. These do not fully reflect the burden of these disorders because 27% of DEEs and 70% of ID+E begin later, with seizure onset after the age of 3 years. Understanding the cumulative incidence of specific syndromes together with the broad group of DEEs is essential for the planning of therapeutic trials. Given trials focus on specific syndromes, there is a risk that effective therapies will not be developed for one-third of children with DEE.
© 2022 American Academy of Neurology.
Conflict of interest statement
G. Poke receives funding from the Health Research Council of New Zealand; J. Stanley receives funding from the Health Research Council of New Zealand; I.E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals. Scheffer has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cereval Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceutical, Zogenix, and Zynerba; has consulted for Atheneum Partners, Care Beyond Diagnosis, Epilepsy Consortium, Ovid Therapeutics, UCB, and Zynerba Pharmaceuticals; is a Nonexecutive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited; may accrue future revenue on pending patent WO61/010,176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies; and has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001,321 (TECH ID:2012-009); L.G. Sadleir receives funding from the Health Research Council of New Zealand and Cure Kids New Zealand; is a consultant for the Epilepsy Consortium; has received travel grants from Seqirus and Nutricia, has received research grants and consultancy fees from Zynerba Pharmaceuticals; and has served on an Eisai Pharmaceuticals scientific advisory panel. Go to
Figures



Comment in
-
Intellectual Disability and Epilepsy: The High Incidence and the Risks of Status Epilepticus and Sudden Death Require Improved Therapies.Epilepsy Curr. 2023 Nov 30;23(6):354-356. doi: 10.1177/15357597231203079. eCollection 2023 Nov-Dec. Epilepsy Curr. 2023. PMID: 38269346 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
