Recent progress in ferroptosis: inducers and inhibitors
- PMID: 36581640
- PMCID: PMC9800531
- DOI: 10.1038/s41420-022-01297-7
Recent progress in ferroptosis: inducers and inhibitors
Erratum in
-
Correction: Recent progress in ferroptosis: inducers and inhibitors.Cell Death Discov. 2023 Apr 17;9(1):130. doi: 10.1038/s41420-023-01431-z. Cell Death Discov. 2023. PMID: 37069156 Free PMC article. No abstract available.
Abstract
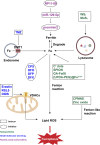
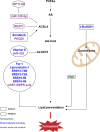
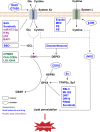
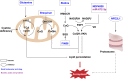
Ferroptosis is a new iron-dependent form of programmed cell death characterized by iron accumulation and lipid peroxidation. In recent years, ferroptosis has garnered enormous interest in disease treatment research communities in pursuit to reveal the mechanism and key targets of ferroptosis because ferroptosis is closely related to the pathophysiological processes of many diseases. Recent studies have shown some key targets, such as glutathione peroxidase 4 (GPX4) and System Xc-, and several inducers and inhibitors have been developed to regulate these key targets. With the emergence of new ferroptosis targets, studies on inducers and inhibitors have made new developments. The selection and use of inducers and inhibitors are very important for related work. This paper briefly introduces important regulatory targets in the ferroptosis metabolic pathway, lists and categorizes commonly used and recently developed inducers and inhibitors, and discusses their medical application. The paper ends of with potential future research direction for ferroptosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review.Ann Transl Med. 2022 Mar;10(6):368. doi: 10.21037/atm-21-6942. Ann Transl Med. 2022. PMID: 35434035 Free PMC article. Review.
-
Ferroptosis: Emerging Role in Diseases and Potential Implication of Bioactive Compounds.Int J Mol Sci. 2023 Dec 8;24(24):17279. doi: 10.3390/ijms242417279. Int J Mol Sci. 2023. PMID: 38139106 Free PMC article. Review.
-
Molecular mechanisms of ferroptosis and their involvement in brain diseases.Pharmacol Ther. 2023 Apr;244:108373. doi: 10.1016/j.pharmthera.2023.108373. Epub 2023 Mar 8. Pharmacol Ther. 2023. PMID: 36894028 Review.
-
Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis.Int J Mol Sci. 2022 Dec 27;24(1):449. doi: 10.3390/ijms24010449. Int J Mol Sci. 2022. PMID: 36613888 Free PMC article. Review.
-
Ferroptosis Inducers for Prostate Cancer Therapy.Curr Med Chem. 2022;29(24):4185-4201. doi: 10.2174/0929867329666220111120924. Curr Med Chem. 2022. PMID: 35021966 Review.
Cited by
-
Harnessing Ferroptosis to Overcome Drug Resistance in Colorectal Cancer: Promising Therapeutic Approaches.Cancers (Basel). 2023 Oct 30;15(21):5209. doi: 10.3390/cancers15215209. Cancers (Basel). 2023. PMID: 37958383 Free PMC article. Review.
-
Physical Exercise-Induced Activation of NRF2 and BDNF as a Promising Strategy for Ferroptosis Regulation in Parkinson's Disease.Neurochem Res. 2024 Jul;49(7):1643-1654. doi: 10.1007/s11064-024-04152-6. Epub 2024 May 24. Neurochem Res. 2024. PMID: 38782838 Review.
-
Cepharanthine hydrochloride: a novel ferroptosis-inducing agent for prostate cancer treatment.Front Pharmacol. 2025 Feb 24;16:1536375. doi: 10.3389/fphar.2025.1536375. eCollection 2025. Front Pharmacol. 2025. PMID: 40066333 Free PMC article.
-
Cannabidiol Is a Potential Inhibitor of Ferroptosis in Human Articular Chondrocytes.J Cell Mol Med. 2025 Jul;29(13):e70592. doi: 10.1111/jcmm.70592. J Cell Mol Med. 2025. PMID: 40576283 Free PMC article.
-
Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11.J Exp Clin Cancer Res. 2024 Apr 13;43(1):112. doi: 10.1186/s13046-024-03039-2. J Exp Clin Cancer Res. 2024. PMID: 38610018 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

