Intravascularly infused extracellular matrix as a biomaterial for targeting and treating inflamed tissues
- PMID: 36581694
- PMCID: PMC10166066
- DOI: 10.1038/s41551-022-00964-5
Intravascularly infused extracellular matrix as a biomaterial for targeting and treating inflamed tissues
Abstract
Decellularized extracellular matrix in the form of patches and locally injected hydrogels has long been used as therapies in animal models of disease. Here we report the safety and feasibility of an intravascularly infused extracellular matrix as a biomaterial for the repair of tissue in animal models of acute myocardial infarction, traumatic brain injury and pulmonary arterial hypertension. The biomaterial consists of decellularized, enzymatically digested and fractionated ventricular myocardium, localizes to injured tissues by binding to leaky microvasculature, and is largely degraded in about 3 d. In rats and pigs with induced acute myocardial infarction followed by intracoronary infusion of the biomaterial, we observed substantially reduced left ventricular volumes and improved wall-motion scores, as well as differential expression of genes associated with tissue repair and inflammation. Delivering pro-healing extracellular matrix by intravascular infusion post injury may provide translational advantages for the healing of inflamed tissues 'from the inside out'.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
KLC and AND hold equity in Ventrix, Inc. KLC is a co-founder, consultant, and board member of Ventrix, Inc. The other authors declare no competing interests.
Figures
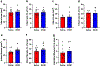
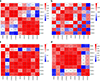
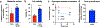






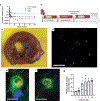
Comment in
-
A biomaterial infused into the bloodstream repairs inflamed tissue.Nat Biomed Eng. 2023 Feb;7(2):92-93. doi: 10.1038/s41551-022-00983-2. Nat Biomed Eng. 2023. PMID: 36627365 No abstract available.
-
Infused ECM targets leaky vessels and heals inflamed tissues.Nat Rev Cardiol. 2023 Mar;20(3):139. doi: 10.1038/s41569-023-00835-9. Nat Rev Cardiol. 2023. PMID: 36650289 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL145709/HL/NHLBI NIH HHS/United States
- F31 HL137347/HL/NHLBI NIH HHS/United States
- R01 HL113468/HL/NHLBI NIH HHS/United States
- T32 HL105373/HL/NHLBI NIH HHS/United States
- R25 HL145817/HL/NHLBI NIH HHS/United States
- T32 HL007444/HL/NHLBI NIH HHS/United States
- R43 HL150917/HL/NHLBI NIH HHS/United States
- F31 HL152610/HL/NHLBI NIH HHS/United States
- T32 GM145304/GM/NIGMS NIH HHS/United States
- F31 HL158212/HL/NHLBI NIH HHS/United States
- R01 HL165232/HL/NHLBI NIH HHS/United States
- F31 HL152686/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

