Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata
- PMID: 36581760
- PMCID: PMC9873571
- DOI: 10.1038/s41477-022-01306-8
Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata
Erratum in
-
Author Correction: Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata.Nat Plants. 2023 Mar;9(3):501. doi: 10.1038/s41477-023-01373-5. Nat Plants. 2023. PMID: 36805039 Free PMC article. No abstract available.
Abstract
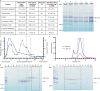
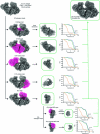
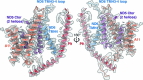
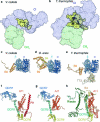
The last steps of cellular respiration-an essential metabolic process in plants-are carried out by mitochondrial oxidative phosphorylation. This process involves a chain of multi-subunit membrane protein complexes (complexes I-V) that form higher-order assemblies called supercomplexes. Although supercomplexes are the most physiologically relevant form of the oxidative phosphorylation complexes, their functions and structures remain mostly unknown. Here we present the cryogenic electron microscopy structure of the supercomplex I + III2 from Vigna radiata (mung bean). The structure contains the full subunit complement of complex I, including a newly assigned, plant-specific subunit. It also shows differences in the mitochondrial processing peptidase domain of complex III2 relative to a previously determined supercomplex with complex IV. The supercomplex interface, while reminiscent of that in other organisms, is plant specific, with a major interface involving complex III2's mitochondrial processing peptidase domain and no participation of complex I's bridge domain. The complex I structure suggests that the bridge domain sets the angle between the enzyme's two arms, limiting large-scale conformational changes. Moreover, complex I's catalytic loops and its response in active-to-deactive assays suggest that, in V. radiata, the resting complex adopts a non-canonical state and can sample deactive- or open-like conformations even in the presence of substrate. This study widens our understanding of the possible conformations and behaviour of complex I and supercomplex I + III2. Further studies of complex I and its supercomplexes in diverse organisms are needed to determine the universal and clade-specific mechanisms of respiration.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Super-complex supercomplex.Nat Plants. 2023 Jan;9(1):5-6. doi: 10.1038/s41477-022-01329-1. Nat Plants. 2023. PMID: 36639556 No abstract available.
References
-
- Nicholls, D. G. & Ferguson, S. J. Bioenergetics 4th edn (Academic Press, 2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

