SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
- PMID: 36581908
- PMCID: PMC9798727
- DOI: 10.1186/s13058-022-01596-y
SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
Abstract
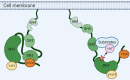
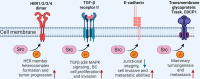
Breast cancer (BC) has been ranked the most common malignant tumor throughout the world and is also a leading cause of cancer-related deaths among women. SRC family kinases (SFKs) belong to the non-receptor tyrosine kinase (nRTK) family, which has eleven members sharing similar structure and function. Among them, SRC is the first identified proto-oncogene in mammalian cells. Oncogenic overexpression or activation of SRC has been revealed to play essential roles in multiple events of BC progression, including tumor initiation, growth, metastasis, drug resistance and stemness regulations. In this review, we will first give an overview of SRC kinase and SRC-relevant functions in various subtypes of BC and then systematically summarize SRC-mediated signaling transductions, with particular emphasis on SRC-mediated substrate phosphorylation in BC. Furthermore, we will discuss the progress of SRC-based targeted therapies in BC and the potential future direction.
Keywords: Breast cancer; SRC kinase; Signaling transduction; Targeted therapy; Tyrosine phosphorylation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

