Highly efficacious and safe neutralizing DNA aptamer of SARS-CoV-2 as an emerging therapy for COVID-19 disease
- PMID: 36581924
- PMCID: PMC9800238
- DOI: 10.1186/s12985-022-01943-7
Highly efficacious and safe neutralizing DNA aptamer of SARS-CoV-2 as an emerging therapy for COVID-19 disease
Abstract
Background: The paucity of SARS-CoV-2-specific virulence factors has greatly hampered the therapeutic management of patients with COVID-19 disease. Although available vaccines and approved therapies have shown tremendous benefits, the continuous emergence of new variants of SARS-CoV-2 and side effects of existing treatments continue to challenge therapy, necessitating the development of a novel effective therapy. We have previously shown that our developed novel single-stranded DNA aptamers not only target the trimer S protein of SARS-CoV-2, but also block the interaction between ACE2 receptors and trimer S protein of Wuhan origin, Delta, Delta plus, Alpha, Lambda, Mu, and Omicron variants of SARS-CoV-2. We herein performed in vivo experiments that administer the aptamer to the lungs by intubation as well as in vitro studies utilizing PBMCs to prove the efficacy and safety of our most effective aptamer, AYA2012004_L.
Methods: In vivo studies were conducted in transgenic mice expressing human ACE2 (K18hACE2), C57BL/6J, and Balb/cJ. Flow cytometry was used to check S-protein expressing pseudo-virus-like particles (VLP) uptake by the lung cells and test the immuogenicity of AYA2012004_L. Ames test was used to assess mutagenicity of AYA2012004_L. RT-PCR and histopathology were used to determine the biodistribution and toxicity of AYA2012004_L in vital organs of mice.
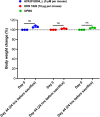
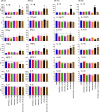
Results: We measured the in vivo uptake of VLPs by lung cells by detecting GFP signal using flow cytometry. AYA2012004_L specifically neutralized VLP uptake and also showed no inflammatory response in mice lungs. In addition, AYA2012004_L did not induce inflammatory response in the lungs of Th1 and Th2 mouse models as well as human PBMCs. AYA2012004_L was detectable in mice lungs and noticeable in insignificant amounts in other vital organs. Accumulation of AYA2012004_L in organs decreased over time. AYA2012004_L did not induce degenerative signs in tissues as seen by histopathology and did not cause changes in the body weight of mice. Ames test also certified that AYA2012004_L is non-mutagenic and proved it to be safe for in vivo studies.
Conclusions: Our aptamer is safe, effective, and can neutralize the uptake of VLPs by lung cells when administered locally suggesting that it can be used as a potential therapeutic agent for COVID-19 management.
Keywords: ACE2; Aptamer; COVID-19; Neutralization; RBD; SARS-CoV-2; Safety; Spike S protein; Toxicity; VLP; Virus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Ledford H. Hundreds of covid trials could provide a deluge of new drugs. Nature 2022;25–7. - PubMed
-
- Information on covid-19 treatment, prevention and research. U.S. Department of Health and Human Services. https://www.covid19treatmentguidelines.nih.gov/
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

