A systematic review and meta-analysis of neoadjuvant chemoimmunotherapy in stage III non-small cell lung cancer
- PMID: 36582007
- PMCID: PMC9798701
- DOI: 10.1186/s12890-022-02292-5
A systematic review and meta-analysis of neoadjuvant chemoimmunotherapy in stage III non-small cell lung cancer
Abstract
Background: Stage III non-small cell lung cancer (NSCLC) is a heterogeneous disease with different subtypes, multidisciplinary teams-led management, and a poor prognosis. Currently, the clinical benefits of stage III NSCLC in the neoadjuvant setting are still unclear. We performed a meta-analysis of published data on neoadjuvant chemoimmunotherapy in stage III NSCLC to systematically evaluate its efficacy and safety.
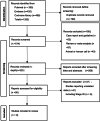
Methods: We searched the databases to identify eligible studies of neoadjuvant chemoimmunotherapy for stage III NSCLC. The primary outcomes mainly included pathological and radiological response outcomes, the feasibility of surgery, and the safety of the regimen. The pathological and radiological response included the rate of major pathologic response (MPR), complete pathologic response (pCR), radiological response outcomes, and R0 resection; The feasibility included the rate of surgical resection, conversion to thoracotomy, surgical complications, pathological downstaging of clinical disease stage. The safety included the incidence of treatment-related adverse events (TRAEs) and severe adverse events (SAEs). R 4.1.3 software was conducted for data analysis, and p < 0.05 was considered statistically significant.
Results: Nine trials containing a total of 382 populations were eligible for the meta-analysis, with the pooled surgical resection rate of 90%. Owing to the large heterogeneity of the single-rate meta-analysis, the random effect model was adopted. The estimated pooled prevalence of MPR was 56% (95%CI 0.39-0.72) and of pCR was 39% (95%CI 0.28-0.51). The pooled rate of TRAEs was 65% (95%CI 0.17-0.99) and SAEs was 24% (95%CI 0.05-0.49).
Conclusion: Compared to neoadjuvant chemotherapy or immunotherapy, neoadjuvant chemoimmunotherapy achieved more pathological and radiological relief, and has a high surgical resection rate and low risk of conversion to thoracotomy and surgical complications, with poor tolerance of toxicity but rarely developing life-threatening adverse events. In conclusion, neoadjuvant chemoimmunotherapy is suggested to be beneficial for stage III NSCLC.
Keywords: Neoadjuvant chemoimmunotherapy; Non-small cell lung cancer; Safety and efficacy; Stage III.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures

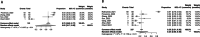

Similar articles
-
A Meta-Analysis of Efficacy and Safety of Neoadjuvant Immunotherapy Plus Chemotherapy for Resectable Non-Small Cell Lung Cancer.Clin Respir J. 2024 Oct;18(10):e70019. doi: 10.1111/crj.70019. Clin Respir J. 2024. PMID: 39359047 Free PMC article.
-
Efficacy and safety of neoadjuvant immunotherapy protocols and cycles for non-small cell lung cancer: a systematic review and meta-analysis.Front Oncol. 2024 Jan 16;14:1276549. doi: 10.3389/fonc.2024.1276549. eCollection 2024. Front Oncol. 2024. PMID: 38292925 Free PMC article.
-
Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis.Transl Lung Cancer Res. 2022 Feb;11(2):277-294. doi: 10.21037/tlcr-22-75. Transl Lung Cancer Res. 2022. PMID: 35280319 Free PMC article.
-
Clinical outcomes of conversion surgery following neoadjuvant chemoimmunotherapy in potentially resectable stage IIIA/IIIB non-small cell lung.Sci Rep. 2025 May 26;15(1):18422. doi: 10.1038/s41598-025-99571-y. Sci Rep. 2025. PMID: 40419636 Free PMC article.
-
Neoadjuvant immunotherapy and chemoimmunotherapy for stage II-III muscle invasive bladder cancer.Front Immunol. 2022 Aug 17;13:986359. doi: 10.3389/fimmu.2022.986359. eCollection 2022. Front Immunol. 2022. PMID: 36059550 Free PMC article.
Cited by
-
Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes.Curr Oncol. 2023 Oct 12;30(10):9104-9115. doi: 10.3390/curroncol30100658. Curr Oncol. 2023. PMID: 37887558 Free PMC article.
-
A Meta-Analysis of Efficacy and Safety of Neoadjuvant Immunotherapy Plus Chemotherapy for Resectable Non-Small Cell Lung Cancer.Clin Respir J. 2024 Oct;18(10):e70019. doi: 10.1111/crj.70019. Clin Respir J. 2024. PMID: 39359047 Free PMC article.
-
Perioperative or neo/adjuvant chemoimmunotherapy versus chemotherapy for resectable non-small cell lung cancer: a systematic review and network meta-analysis.Syst Rev. 2025 Jan 24;14(1):24. doi: 10.1186/s13643-025-02767-6. Syst Rev. 2025. PMID: 39856765 Free PMC article.
-
Potential predictors of the pathologic response after neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer: a narrative review.Transl Lung Cancer Res. 2024 May 31;13(5):1137-1149. doi: 10.21037/tlcr-24-142. Epub 2024 May 24. Transl Lung Cancer Res. 2024. PMID: 38854945 Free PMC article. Review.
-
The Value of Perioperative Immunotherapy for Non-Small Cell Lung Cancer: A Pool- and Meta-Analysis.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241258164. doi: 10.1177/15330338241258164. Technol Cancer Res Treat. 2024. PMID: 38872482 Free PMC article.
References
-
- Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, Rodríguez-Martínez Á, Giraldo-Osorioet A, Varela-Lema L, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021;10(1):506–18. doi: 10.21037/tlcr.2020.03.40. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. V. 2. 2014. Available online http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
-
- The American Joint Committee on Cancer . AJCC cancer staging manual. 8. Chicago: AJCC; 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

