Organelle-targeted biosensors reveal distinct oxidative events during pattern-triggered immune responses
- PMID: 36582183
- PMCID: PMC10069903
- DOI: 10.1093/plphys/kiac603
Organelle-targeted biosensors reveal distinct oxidative events during pattern-triggered immune responses
Abstract
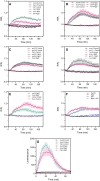
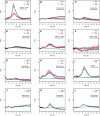
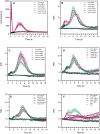
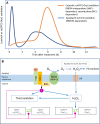
Reactive oxygen species are produced in response to pathogens and pathogen-associated molecular patterns, as exemplified by the rapid extracellular oxidative burst dependent on the NADPH oxidase isoform RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) in Arabidopsis (Arabidopsis thaliana). We used the H2O2 biosensor roGFP2-Orp1 and the glutathione redox state biosensor GRX1-roGFP2 targeted to various organelles to reveal unsuspected oxidative events during the pattern-triggered immune response to flagellin (flg22) and after inoculation with Pseudomonas syringae. roGFP2-Orp1 was oxidized in a biphasic manner 1 and 6 h after treatment, with a more intense and faster response in the cytosol compared to chloroplasts, mitochondria, and peroxisomes. Peroxisomal and cytosolic GRX1-roGFP2 were also oxidized in a biphasic manner. Interestingly, our results suggested that bacterial effectors partially suppress the second phase of roGFP2-Orp1 oxidation in the cytosol. Pharmacological and genetic analyses indicated that the pathogen-associated molecular pattern-induced cytosolic oxidation required the BRI1-ASSOCIATED RECEPTOR KINASE (BAK1) and BOTRYTIS-INDUCED KINASE 1 (BIK1) signaling components involved in the immune response but was largely independent of NADPH oxidases RBOHD and RESPIRATORY BURST OXIDASE HOMOLOG F (RBOHF) and apoplastic peroxidases peroxidase 33 (PRX33) and peroxidase 34 (PRX34). The initial apoplastic oxidative burst measured with luminol was followed by a second oxidation burst, both of which preceded the two waves of cytosolic oxidation. In contrast to the cytosolic oxidation, these bursts were RBOHD-dependent. Our results reveal complex oxidative sources and dynamics during the pattern-triggered immune response, including that cytosolic oxidation is largely independent of the preceding extracellular oxidation events.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



References
-
- Albrecht SC, Sobotta MC, Bausewein D, Aller I, Hell R, Dick TP, Meyer AJ (2014) Redesign of genetically encoded biosensors for monitoring mitochondrial redox status in a broad range of model eukaryotes. J Biomol Screen 19(3): 379–386 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

