The role of immune cells in modulating chronic inflammation and osteonecrosis
- PMID: 36582244
- PMCID: PMC9792770
- DOI: 10.3389/fimmu.2022.1064245
The role of immune cells in modulating chronic inflammation and osteonecrosis
Abstract
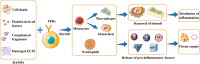
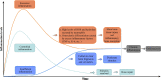
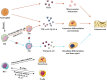
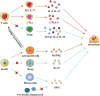
Osteonecrosis occurs when, under continuous stimulation by adverse factors such as glucocorticoids or alcohol, the death of local bone and marrow cells leads to abnormal osteoimmune function. This creates a chronic inflammatory microenvironment, which interferes with bone regeneration and repair. In a variety of bone tissue diseases, innate immune cells and adaptive immune cells interact with bone cells, and their effects on bone metabolic homeostasis have attracted more and more attention, thus developing into a new discipline - osteoimmunology. Immune cells are the most important regulator of inflammation, and osteoimmune disorder may be an important cause of osteonecrosis. Elucidating the chronic inflammatory microenvironment regulated by abnormal osteoimmune may help develop potential treatments for osteonecrosis. This review summarizes the inflammatory regulation of bone immunity in osteonecrosis, explains the pathophysiological mechanism of osteonecrosis from the perspective of osteoimmunology, and provides new ideas for the treatment of osteonecrosis.
Keywords: bone regeneration; cytokines; immune cells; inflammation; osteoimmunology; osteonecrosis.
Copyright © 2022 Zheng, Yao, Xue, Wang and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Unraveling the Role of Endothelial Dysfunction in Osteonecrosis of the Femoral Head: A Pathway to New Therapies.Biomedicines. 2024 Mar 15;12(3):664. doi: 10.3390/biomedicines12030664. Biomedicines. 2024. PMID: 38540277 Free PMC article. Review.
-
[Role and mechanism of macrophage-mediated osteoimmune in osteonecrosis of the femoral head].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Jan 15;38(1):119-124. doi: 10.7507/1002-1892.202308026. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38225851 Free PMC article. Chinese.
-
Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity.Nat Rev Rheumatol. 2018 Mar;14(3):146-156. doi: 10.1038/nrrheum.2017.213. Epub 2018 Jan 11. Nat Rev Rheumatol. 2018. PMID: 29323344 Free PMC article. Review.
-
Inflammation, Bone Healing and Osteonecrosis: From Bedside to Bench.J Inflamm Res. 2020 Nov 13;13:913-923. doi: 10.2147/JIR.S281941. eCollection 2020. J Inflamm Res. 2020. PMID: 33223846 Free PMC article. Review.
-
Alcoholism and Osteoimmunology.Curr Med Chem. 2021;28(9):1815-1828. doi: 10.2174/1567201816666190514101303. Curr Med Chem. 2021. PMID: 32334496 Review.
Cited by
-
Recent Advances in Nanotechnology-Based Strategies for Bone Tuberculosis Management.Pharmaceuticals (Basel). 2024 Jan 29;17(2):170. doi: 10.3390/ph17020170. Pharmaceuticals (Basel). 2024. PMID: 38399384 Free PMC article. Review.
-
PDGF-BB improves cortical bone quality through restoring the osteogenic microenvironment in the steroid-associated osteonecrosis of rabbits.J Orthop Translat. 2025 Apr 12;52:97-115. doi: 10.1016/j.jot.2025.03.010. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40275883 Free PMC article.
-
Gut microbiota and osteonecrosis: A Mendelian randomization study.Medicine (Baltimore). 2025 Mar 7;104(10):e41703. doi: 10.1097/MD.0000000000041703. Medicine (Baltimore). 2025. PMID: 40068074 Free PMC article.
-
Risk factors for avascular necrosis in patients with systemic lupus erythematosus: a multi-center cohort study of Chinese SLE Treatment and Research Group (CSTAR) Registry XXII.Arthritis Res Ther. 2023 May 12;25(1):78. doi: 10.1186/s13075-023-03061-3. Arthritis Res Ther. 2023. PMID: 37173771 Free PMC article.
-
Unraveling the Role of Endothelial Dysfunction in Osteonecrosis of the Femoral Head: A Pathway to New Therapies.Biomedicines. 2024 Mar 15;12(3):664. doi: 10.3390/biomedicines12030664. Biomedicines. 2024. PMID: 38540277 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

