Dynamics of circulating follicular helper T cell subsets and follicular regulatory T cells in rheumatoid arthritis patients according to HLA-DRB1 locus
- PMID: 36582249
- PMCID: PMC9793086
- DOI: 10.3389/fimmu.2022.1000982
Dynamics of circulating follicular helper T cell subsets and follicular regulatory T cells in rheumatoid arthritis patients according to HLA-DRB1 locus
Abstract
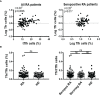
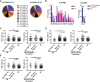
B cells, follicular helper T (Tfh) cells and follicular regulatory T (Tfr) cells are part of a circuit that may play a role in the development or progression of rheumatoid arthritis (RA). With the aim of providing further insight into this topic, here we evaluated the frequency of different subsets of Tfh and Tfr in untreated and long-term treated RA patients from a cohort of Argentina, and their potential association with particular human leukocyte antigen (HLA) class-II variants and disease activity. We observed that the frequency of total Tfh cells as well as of particular Tfh subsets and Tfr cells were increased in seropositive untreated RA patients. Interestingly, when analyzing paired samples, the frequency of Tfh cells was reduced in synovial fluid compared to peripheral blood, while Tfr cells levels were similar in both biological fluids. After treatment, a decrease in the CCR7loPD1hi Tfh subset and an increase in the frequency of Tfr cells was observed in blood. In comparison to healthy donors, seropositive patients with moderate and high disease activity exhibited higher frequency of Tfh cells while seropositive patients with low disease activity presented higher Tfr cell frequency. Finally, we observed that HLA-DRB1*09 presence correlated with higher frequency of Tfh and Tfr cells, while HLA-DRB1*04 was associated with increased Tfr cell frequency. Together, our results increase our knowledge about the dynamics of Tfh and Tfr cell subsets in RA, showing that this is altered after treatment.
Keywords: DAS28-ESR; DMARDs; HLA-DRB1; Tfh cells; Tfr cells; rheumatoid arthritis.
Copyright © 2022 Ferrero, Onofrio, Acosta, Zacca, Ponce, Mussano, Onetti, Cadile, Costantino, Werner, Mas, Alvarellos, Montes, Acosta Rodríguez and Gruppi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

