Smart dental materials for antimicrobial applications
- PMID: 36582351
- PMCID: PMC9763696
- DOI: 10.1016/j.bioactmat.2022.12.002
Smart dental materials for antimicrobial applications
Abstract
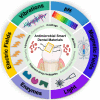
Smart biomaterials can sense and react to physiological or external environmental stimuli (e.g., mechanical, chemical, electrical, or magnetic signals). The last decades have seen exponential growth in the use and development of smart dental biomaterials for antimicrobial applications in dentistry. These biomaterial systems offer improved efficacy and controllable bio-functionalities to prevent infections and extend the longevity of dental devices. This review article presents the current state-of-the-art of design, evaluation, advantages, and limitations of bioactive and stimuli-responsive and autonomous dental materials for antimicrobial applications. First, the importance and classification of smart biomaterials are discussed. Second, the categories of bioresponsive antibacterial dental materials are systematically itemized based on different stimuli, including pH, enzymes, light, magnetic field, and vibrations. For each category, their antimicrobial mechanism, applications, and examples are discussed. Finally, we examined the limitations and obstacles required to develop clinically relevant applications of these appealing technologies.
Keywords: Antibacterial; Antibiofilm; Antifungal; Antimicrobial; Bioactive; Biofilm; Bioresponsive biomaterials; Restorative dentistry; Smart dental materials; Stimuli-responsive.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Tjäderhane L., et al. Dentin basic structure and composition—an overview. Endod. Top. 2009;20:3–29. doi: 10.1111/j.1601-1546.2012.00269.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

