Regional specialization of the tongue revealed by gustatory ganglion imaging
- PMID: 36582484
- PMCID: PMC9792408
- DOI: 10.1016/j.isci.2022.105700
Regional specialization of the tongue revealed by gustatory ganglion imaging
Abstract
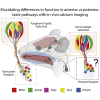
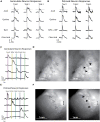
Gustatory information is relayed from the anterior tongue by geniculate ganglion neurons and from the posterior tongue by neurons of the petrosal portion of the jugular/nodose/petrosal ganglion complex. Here, we use in vivo calcium imaging in mice to compare the encoding of taste information in the geniculate and petrosal ganglia, at single-neuron resolution. Our data support an anterior/posterior specialization of taste information coding from the tongue to the ganglia, with petrosal neurons more responsive to umami or bitter and less responsive to sweet or salty stimuli than geniculate neurons. We found that umami (50 mM MPG + 1 mM IMP) promotes salivation when applied to the posterior, but not anterior, tongue. This suggests a functional taste map of the mammalian tongue where the anterior and posterior taste pathways are differentially responsive to specific taste qualities, and differentially regulate downstream physiological functions of taste, such as promoting salivation.
Keywords: Cellular neuroscience; sensory neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bartoshuk L.M. The biological basis of food perception and acceptance. Food Qual. Prefer. 1993;4:21–32.
-
- Boring E. 1942. Sensation and Perception in the History of Psychology.
-
- Hanig D.P. Universitat Leipzig; 1901. Psychophysik des Geschmackssinnes. PhD.
-
- Rothova M., Thompson H., Lickert H., Tucker A.S. Lineage tracing of the endoderm during oral development. Dev. Dyn. 2012;241:1183–1191. - PubMed
-
- Frank M.E. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J. Neurophysiol. 1991;65:1452–1463. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

