Study on promoting the regeneration of grafted fat by cell-assisted lipotransfer
- PMID: 36582606
- PMCID: PMC9762074
- DOI: 10.1016/j.reth.2022.11.008
Study on promoting the regeneration of grafted fat by cell-assisted lipotransfer
Abstract
Background: Cell-assisted lipotransfer (CAL), a modified adipose-derived stromal/stem cells (ADSCs)-based approach for autologous fat grafting that is an ideal option for soft tissue augmentation, has many shortcomings in terms of retention and adverse effects. The objective of our study was to improve the treatment efficacy of CAL by adding fibroblasts.
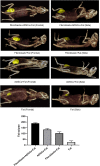
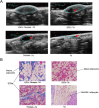
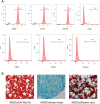
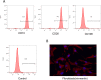
Methods: ADSCs and fibroblasts were isolated from human adipose and dermal tissues, with fibroblasts identified by immunofluorescence and ADSCs identified by the multilineage differentiation method. We performed cell proliferation, apoptosis, migration, adipogenic, and hemangioendothelial differentiation experiments, qPCR and Western blotting analysis in co-cultures of fibroblasts and ADSCs. Subsequently, we conducted animal experiments with BALB/c nude mice. Masson's staining, immunofluorescence staining and ultrasound were used to analyze the occurrence of adverse reactions of the grafted fat, and CT and three-dimensional reconstruction were used to accurately evaluate the volume of the grafted fat.
Results: We found that the co-culture of fibroblasts and ADSCs promoted their mutual proliferation, adipogenic differentiation, hemangioendothelial differentiation and proliferation and migration of HUVECs. Fibroblasts inhibit the apoptosis of ADSCs. Moreover, in animal experiments, the autografted adipose group combined with ADSCs and fibroblasts had the least occurrence of oily cysts, and fat had the best form of survival.
Conclusions: We enhanced adipocyte regeneration and angiogenesis in ADSCs and fibroblast cells after adding fibroblasts to conventional CAL autologous fat grafts. In turn, the volume retention rate of the grafted fat is improved, and the adverse reactions are reduced.
Keywords: Adipose-derived stromal/stem cells; Autologous fat grafting; Cell-assisted lipotransfer; Fibroblasts.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Biological characteristics of adipose-derived stem cells from patients with progressive hemifacial atrophy: An in vivo study.J Cosmet Dermatol. 2022 Nov;21(11):6134-6144. doi: 10.1111/jocd.15162. Epub 2022 Jun 26. J Cosmet Dermatol. 2022. PMID: 35708514
-
Novel breast reconstruction technique using ex vivo mononuclear (RE-01) cells and adipose-derived mesenchymal stem cells.Regen Ther. 2025 Mar 29;29:271-281. doi: 10.1016/j.reth.2025.03.018. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40230355 Free PMC article.
-
[Effects of adipose-derived stem cells and endothelial cells on survival and neovascularization of fat tissue transplants].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Aug 15;32(8):1074-1080. doi: 10.7507/1002-1892.201802069. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 30238738 Free PMC article. Chinese.
-
[Advancement of adipose-derived stem cells assisted autologous lipotransfer in breast repair and reconstruction].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Oct;27(10):1252-5. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24397141 Review. Chinese.
-
Cell-assisted lipotransfer: Friend or foe in fat grafting? Systematic review and meta-analysis.J Tissue Eng Regen Med. 2018 Feb;12(2):e1237-e1250. doi: 10.1002/term.2524. Epub 2017 Dec 7. J Tissue Eng Regen Med. 2018. PMID: 28719946
Cited by
-
Adipose-Derived Stem Cells: Angiogenetic Potential and Utility in Tissue Engineering.Int J Mol Sci. 2024 Feb 16;25(4):2356. doi: 10.3390/ijms25042356. Int J Mol Sci. 2024. PMID: 38397032 Free PMC article. Review.
-
Deconstructing Fat to Reverse Radiation Induced Soft Tissue Fibrosis.Bioengineering (Basel). 2023 Jun 20;10(6):742. doi: 10.3390/bioengineering10060742. Bioengineering (Basel). 2023. PMID: 37370673 Free PMC article. Review.
-
NPTX1 Mediates the Facilitating Effects of Hypoxia-Stimulated Human Adipocytes on Adipose-Derived Stem Cell Activation and Autologous Adipose Graft Survival Rate.Aesthetic Plast Surg. 2024 Oct;48(20):4203-4216. doi: 10.1007/s00266-024-04118-7. Epub 2024 May 24. Aesthetic Plast Surg. 2024. PMID: 38789811
References
-
- Blondeel P.N., Hijjawi J., Depypere H., Roche N., Van Landuyt K. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg. 2009;123(2):455–462. - PubMed
-
- Kolle S.T., Duscher D., Taudorf M., Fischer-Nielsen A., Svalgaard J.D., Munthe-Fog L., et al. Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial. Stem Cells Transl Med. 2020;9(11):1277–1286. - PMC - PubMed
LinkOut - more resources
Full Text Sources

