Biomarker-driven drug repurposing on biologically similar cancers with DNA-repair deficiencies
- PMID: 36583025
- PMCID: PMC9792769
- DOI: 10.3389/fgene.2022.1015531
Biomarker-driven drug repurposing on biologically similar cancers with DNA-repair deficiencies
Abstract
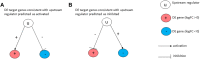
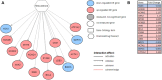
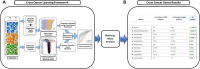
Similar molecular and genetic aberrations among diseases can lead to the discovery of jointly important treatment options across biologically similar diseases. Oncologists closely looked at several hormone-dependent cancers and identified remarkable pathological and molecular similarities in their DNA repair pathway abnormalities. Although deficiencies in Homologous Recombination (HR) pathway plays a significant role towards cancer progression, there could be other DNA-repair pathway deficiencies that requires careful investigation. In this paper, through a biomarker-driven drug repurposing model, we identified several potential drug candidates for breast and prostate cancer patients with DNA-repair deficiencies based on common specific biomarkers and irrespective of the organ the tumors originated from. Normalized discounted cumulative gain (NDCG) and sensitivity analysis were used to assess the performance of the drug repurposing model. Our results showed that Mitoxantrone and Genistein were among drugs with high therapeutic effects that significantly reverted the gene expression changes caused by the disease (FDR adjusted p-values for prostate cancer =1.225e-4 and 8.195e-8, respectively) for patients with deficiencies in their homologous recombination (HR) pathways. The proposed multi-cancer treatment framework, suitable for patients whose cancers had common specific biomarkers, has the potential to identify promising drug candidates by enriching the study population through the integration of multiple cancers and targeting patients who respond poorly to organ-specific treatments.
Keywords: DNA repair; drug repurposing; homologous recombination; mitoxantrone; multi cancer treatment; personalized medicine.
Copyright © 2022 Munj, Taz, Arslanturk and Heath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Beaver J. A., Amiri-Kordestani L., Charlab R., Chen W., Palmby T., Tilley A., et al. (2015). Fda approval: Palbociclib for the treatment of postmenopausal patients with estrogen receptor–positive, her2-negative metastatic breast cancer. Clin. Cancer Res. 21, 4760–4766. 10.1158/1078-0432.CCR-15-1185 - DOI - PubMed
LinkOut - more resources
Full Text Sources

