Bonding of Neuropeptide Y on Graphene Oxide for Drug Delivery Applications to the Central Nervous System
- PMID: 36583122
- PMCID: PMC9791619
- DOI: 10.1021/acsanm.2c03409
Bonding of Neuropeptide Y on Graphene Oxide for Drug Delivery Applications to the Central Nervous System
Erratum in
-
Correction to "Bonding of Neuropeptide Y on Graphene Oxide for Drug Delivery Applications to the Central Nervous System".ACS Appl Nano Mater. 2023 May 2;6(9):8105. doi: 10.1021/acsanm.3c01600. eCollection 2023 May 12. ACS Appl Nano Mater. 2023. PMID: 37205294 Free PMC article.
Abstract
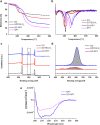
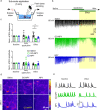
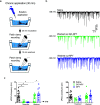
Nanoscale graphene-based materials (GBMs) enable targeting subcellular structures of the nervous system, a feature crucial for the successful engineering of alternative nanocarriers to deliver drugs and to treat neurodisorders. Among GBMs, graphene oxide (GO) nanoflakes, showing good dispersibility in water solution and being rich of functionalizable oxygen groups, are ideal core structures for carrying biological active molecules to the brain, such as the neuropeptide Y (NPY). In addition, when unconjugated, these nanomaterials have been reported to modulate neuronal function per se. Although some GBM-based nanocarriers have been tested both in vitro and in vivo, a thorough characterization of covalent binding impact on the biological properties of the carried molecule and/or of the nanomaterial is still missing. Here, a copper(I)-catalyzed alkyne-azide cycloaddition strategy was employed to synthesize the GO-NPY complex. By investigating through electrophysiology the impact of these conjugates on the activity of hippocampal neurons, we show that the covalent modification of the nanomaterial, while making GO an inert platform for the vectorized delivery, enhances the duration of NPY pharmacological activity. These findings support the future use of GO for the development of smart platforms for nervous system drug delivery.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
