Gating with Charge Inversion to Control Ionic Transport in Nanopores
- PMID: 36583126
- PMCID: PMC9791654
- DOI: 10.1021/acsanm.2c03573
Gating with Charge Inversion to Control Ionic Transport in Nanopores
Abstract
Multivalent ions modify the properties of the solid/liquid interfaces, and in some cases, they can even invert the polarity of surface charge, having large consequences for separation processes based on charge. The so-called charge inversion is observed as a switch from negative surface charge in monovalent salts, e.g., KCl, to effective positive surface charge in multivalent salts that is possible through a strong accumulation and correlation of the multivalent ions at the surface. It is not known yet, however, whether the density of the positive charge induced by charge inversion depends on the pore opening diameter, especially in extreme nanoconfinement. Here, we probe how the effective surface charge induced by charge inversion is influenced by the pore opening diameter using a series of nanopores with an opening between 4 and 25 nm placed in contact with trivalent chromium ions in tris(ethylenediamine)chromium(III) sulfate at different concentrations. Our results suggest that the effective positive charge density can indeed be modified by nanoconfinement to the extent that is dependent on the pore diameter, salt concentration, and applied voltage. In addition, the correlated ions can increase the transmembrane current in nanopores with an opening diameter down to 10 nm and cause a significant blockage of the current for narrower pores. The results provide guidelines to control ionic transport at the nanoscale with multivalent ions and demonstrate that in the same experimental conditions, differently sized pores in the same porous material can feature different surface charge density and possibly ion selectivity.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

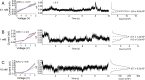

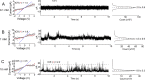

References
-
- Grosberg A. Y.; Nguyen T. T.; Shklovskii B. I. Colloquium: The Physics of Charge Inversion in Chemical and Biological Systems. Rev. Mod. Phys. 2002, 74, 329–345. 10.1103/RevModPhys.74.329. - DOI
-
- Besteman K.; Van Eijk K.; Lemay S. G. Charge Inversion Accompanies DNA Condensation by Multivalent Ions. Nature Phys. 2007, 3, 641–644. 10.1038/nphys697. - DOI
-
- Wernersson E.; Kjellander R.; Lyklema J. Charge Inversion and Ion–Ion Correlation Effects at the Mercury/Aqueous MgSO4 Interface: Toward the Solution of a Long-Standing Issue. J. Phys. Chem. C 2010, 114, 1849–1866. 10.1021/jp906759e. - DOI
LinkOut - more resources
Full Text Sources
