Estimating the effect of HIV on cervical cancer elimination in South Africa: Comparative modelling of the impact of vaccination and screening
- PMID: 36583170
- PMCID: PMC9793279
- DOI: 10.1016/j.eclinm.2022.101754
Estimating the effect of HIV on cervical cancer elimination in South Africa: Comparative modelling of the impact of vaccination and screening
Abstract
Background: In 2020, the World Health Organization (WHO) launched its initiative to eliminate cervical cancer as a public health problem. To inform global efforts for countries with high HIV and cervical cancer burden, we assessed the impact of human papillomavirus (HPV) vaccination and cervical cancer screening and treatment in South Africa, on cervical cancer and the potential for achieving elimination before 2120, considering faster HPV disease progression and higher cervical cancer risk among women living with HIV(WLHIV) and HIV interventions.
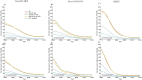
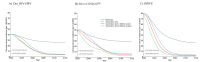
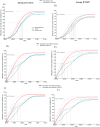
Methods: Three independent transmission-dynamic models simulating HIV and HPV infections and disease progression were used to predict the impact on cervical cancer incidence of three scenarios for all women: 1) girls' vaccination (9-14 years old), 2) girls' vaccination plus 1 lifetime cervical screen (at 35 years), and 3) girls' vaccination plus 2 lifetime cervical screens (at 35 and 45 years) and three enhanced scenarios for WLHIV: 4) vaccination of young WLHIV aged 15-24 years, 5) three-yearly cervical screening of WLHIV aged 15-49 years, or 6) both. Vaccination assumed 90% coverage and 100% lifetime protection with the nonavalent vaccine (against HPV-16/18/31/33/45/52/58). Cervical cancer screening assumed HPV testing with uptake increasing from 45% (2023), 70% (2030) to 90% (2045+). We also assumed that UNAIDS 90-90-90 HIV treatment and 70% male circumcision targets are reached by 2030. We examined three elimination thresholds: age-standardised cervical cancer incidence rates below 4 or 10 per 100,000 women-years, and >85% reduction in cervical cancer incidence rate. We conducted sensitivity analyses and presented the median age-standardised predictions of outcomes of the three models (minimum-maximum across models).
Findings: Girls' vaccination could reduce age-standardised cervical cancer incidence from a median of 47.6 (40.9-79.2) in 2020 to 4.5 (3.2-6.3) per 100,000 women-years by 2120, averting on average ∼4% and ∼46% of age-standardised cumulative cervical cancer cases over 25 and 100 years, respectively, compared to the basecase. Adding 2 lifetime screens helped achieve elimination over the century among all women (2120 cervical cancer incidence: 3.6 (1.9-3.6) per 100,000 women-years), but not among WLHIV (10.8 (5.3-11.6)), and averted more cumulative cancer cases overall (∼45% over 25 years and ∼61% over 100 years compared to basecase) than girls' vaccination alone. Adding three-yearly cervical screening among WLHIV (to girls' vaccination and 2 lifetime cervical screens) further reduced age-standardised cervical cancer incidence to 3.3 (1.8-3.6) per 100,000 women-years overall and to 5.2 (3.9-8.5) among WLHIV by 2120 and averted on average 12-13% additional cumulative cancer cases among all women and 21-24% among WLHIV than girls' vaccination and 2 lifetime cervical screens over 25 years or longer. Long-term vaccine protection and using the nonavalent vaccine was required for elimination.
Interpretation: High HPV vaccination coverage of girls and 2 lifetime cervical screens could eliminate cervical cancer among women overall in South Africa by the end of the century and substantially decrease cases among all women and WLHIV over the short and medium term. Cervical cancer elimination in WLHIV would likely require enhanced prevention strategies for WLHIV. Screening of WLHIV remains an important strategy to reduce incidence and alleviate disparities in cervical cancer burden between women with and without HIV, despite HIV interventions scale-up.
Funding: World Health Organization. National Cancer Institute, National Institutes of Health. MRC Centre for Global Infectious Disease Analysis, UK Medical Research Council. National Institute of Child Health and Human Development research. Cancer Association of South Africa. Canadian Institutes of Health Research and the Fonds de recherche du Québec - Santé research.
Keywords: Cervical cancer elimination; HIV; HPV vaccination; South Africa; cervical cancer screening.
© 2022 The Authors.
Conflict of interest statement
RVB reports grants from the Bill and Melinda Gates Foundation (BMGF), the 10.13039/100000002National Institutes of Health (10.13039/100000002NIH), and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work. MMR reports funding from Harvard Data Science Institute and travel support to attend meetings for cervical cancer elimination from the WHO and Canadian Institute of Health Research, all outside of the submitted work. CJB did a Graduate Research Assistantship with Merck & Co., Inc. after and outside of the submitted work. GL started working from Merck in March 2022 and detains Merck stock/stock options. MB received funding from the 10.13039/100012016Institut National de Santé Publique du Québec for other work. DWR reports additional NIH, USAID and WHO salary support for unrelated work. LS received funding from 10.13039/100014588Sanofi Pasteur for projects outside the scope of this manuscript. PB, SG, NS, RS, LFJ, SG, and NB declare no competing interests.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Mboumba Bouassa R.S., Prazuck T., Lethu T., Meye J.F., Bélec L. Cervical cancer in sub-Saharan Africa: an emerging and preventable disease associated with oncogenic human papillomavirus. Med Sante Trop. 2017;27(1):16–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

