Ex vivo evaluation of antibiotic sensitivity in samples from endodontic infections
- PMID: 36583208
- PMCID: PMC9793940
- DOI: 10.1080/20002297.2022.2160536
Ex vivo evaluation of antibiotic sensitivity in samples from endodontic infections
Abstract
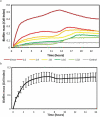
Objective: To develop an in vitro model for real-time monitoring of endodontic biofilm growth and evaluate the ex vivo effect of antibiotics on biofilm growth.
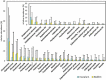
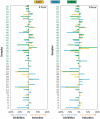
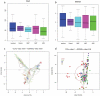
Material and methods: Root canal samples were taken from 40 patients and inoculated into 96-well plates in a system that measures biofilm growth through electrical impedance. Biofilm bacterial composition at the genus and species level was analyzed by Illumina sequencing. ANCOM-BC corrected data were used to compare bacterial composition after antibiotic treatment through compositional analysis, and to compare microbiological with clinical data.
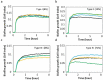
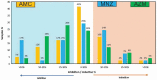
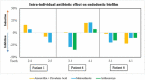
Results: The stationary phase was reached at 8 hours. The biofilm formed had a similar bacterial composition to the inoculum, and Enterococcus faecalis was virtually absent from the samples. The bacterial composition and the effect of antibiotics were sample-dependent. Metronidazole was the antibiotic that most inhibited biofilm formation and azithromycin the one that inhibited it in the highest percentage of cases. The antibiotic effect could not be related to the biofilm original bacterial composition.
Conclusions: The impedance system allowed real-time monitoring of endodontic biofilm formation, and we propose it as a model for ex vivo evaluation of the whole biofilm susceptibility to antimicrobials, as opposed to evaluating antibiotic sensitivity of specific bacterial isolates.
Keywords: Antibiotic; biofilm; biofilm model; endodontic; real time cell analysis.
© 2022 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Characterization and ex vivo modelling of endodontic infections from the Arabian Gulf region.Int Endod J. 2025 Jul;58(7):1091-1108. doi: 10.1111/iej.14227. Epub 2025 Mar 26. Int Endod J. 2025. PMID: 40135668 Free PMC article.
-
Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic infections associated with root-filled teeth.J Endod. 2014 Feb;40(2):223-30. doi: 10.1016/j.joen.2013.07.023. Epub 2013 Sep 7. J Endod. 2014. PMID: 24461408
-
Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model.Photodiagnosis Photodyn Ther. 2020 Mar;29:101643. doi: 10.1016/j.pdpdt.2019.101643. Epub 2019 Dec 30. Photodiagnosis Photodyn Ther. 2020. PMID: 31899382
-
Biofilm model systems for root canal disinfection: a literature review.Int Endod J. 2019 May;52(5):604-628. doi: 10.1111/iej.13050. Epub 2018 Dec 20. Int Endod J. 2019. PMID: 30488449 Review.
-
New Bacterial Combinations in Secondary Endodontic Infections of Patients with a Recent Systematic Antibiotic Therapy.Monogr Oral Sci. 2021;29:144-154. doi: 10.1159/000510190. Epub 2020 Dec 21. Monogr Oral Sci. 2021. PMID: 33427215 Review.
Cited by
-
Personalized antibiotic selection in periodontal treatment improves clinical and microbiological outputs.Front Cell Infect Microbiol. 2023 Dec 18;13:1307380. doi: 10.3389/fcimb.2023.1307380. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38179425 Free PMC article. Clinical Trial.
References
-
- Siqueira Jr JF, Roças IN.. Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J. 2011;37(3):92–99. - PubMed
-
- Ricucci D, Siqueira JF. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod. 2010;36(8):1277–1288. - PubMed
LinkOut - more resources
Full Text Sources
