LncRNA TCF7 contributes to high glucose-induced damage in human podocytes by up-regulating SEMA3A via sponging miR-16-5p
- PMID: 36583231
- PMCID: PMC9889678
- DOI: 10.1111/jdi.13904
LncRNA TCF7 contributes to high glucose-induced damage in human podocytes by up-regulating SEMA3A via sponging miR-16-5p
Abstract
Aims/introduction: Long non-coding RNAs (lncRNAs) exert essential functions in the pathogenesis of diabetic nephropathy (DN). LncRNA T-cell factor 7 (TCF7) and semaphorin-3A (SEMA3A) have been found to be involved in the progression of diabetic nephropathy. However, whether the effect of TCF7 on the pathogenesis of diabetic nephropathy is mediated by SEMA3A remains unclear.
Materials and methods: TCF7, miR-16-5p, and SEMA3A were quantified by a qRT-PCR or immunoblotting method. A CCK-8 assay gauged the cell viability. Measurement of cell apoptosis was done using flow cytometry. RNA immunoprecipitation (RIP), dual-luciferase reporter, and RNA pull-down assays were utilized to assay the targeted interactions among the variables.
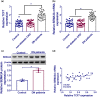
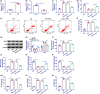
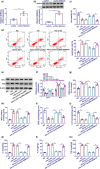
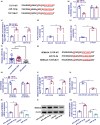
Results: The TCF7 and SEMA3A levels were elevated in serum from patients with diabetic nephropathy. TCF7 silencing or SEMA3A depletion ameliorated high glucose (HG)-induced podocyte injury. Moreover, TCF7 silencing protected against HG-induced podocyte injury by down-regulating SEMA3A. TCF7 targeted miR-16-5p, and miR-16-5p targeted SEMA3A. Furthermore, TCF7 affected the expression of SEMA3A by competing specifically for shared miR-16-5p.
Conclusions: These findings suggested that TCF7 silencing attenuated high glucose-induced podocyte damage partially through the miR-16-5p/SEMA3A regulation cascade.
Keywords: Diabetic nephropathy; Podocyte injury; TCF7.
© 2022 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures


Similar articles
-
Role of Semaphorin 3A in Kidney Development and Diseases.Diagnostics (Basel). 2023 Sep 25;13(19):3038. doi: 10.3390/diagnostics13193038. Diagnostics (Basel). 2023. PMID: 37835781 Free PMC article. Review.
-
KCNQ1OT1 inhibition alleviates high glucose-induced podocyte injury by adsorbing miR-23b-3p and regulating Sema3A.Clin Exp Nephrol. 2022 May;26(5):385-397. doi: 10.1007/s10157-021-02173-x. Epub 2022 Jan 8. Clin Exp Nephrol. 2022. PMID: 34997887
-
miR-15b-5p ameliorated high glucose-induced podocyte injury through repressing apoptosis, oxidative stress, and inflammatory responses by targeting Sema3A.J Cell Physiol. 2019 Nov;234(11):20869-20878. doi: 10.1002/jcp.28691. Epub 2019 Apr 25. J Cell Physiol. 2019. PMID: 31025335
-
Long non-coding RNA Gm11974 aggravates oxygen-glucose deprivation-induced injury via miR-122-5p/SEMA3A axis in ischaemic stroke.Metab Brain Dis. 2021 Oct;36(7):2059-2069. doi: 10.1007/s11011-021-00792-7. Epub 2021 Aug 2. Metab Brain Dis. 2021. PMID: 34338972
-
Roles of transcriptional factor 7 in production of inflammatory factors for lung diseases.J Transl Med. 2015 Aug 20;13:273. doi: 10.1186/s12967-015-0617-7. J Transl Med. 2015. PMID: 26289446 Free PMC article. Review.
Cited by
-
Silencing LncRNA SNHG14 alleviates renal tubular injury via the miR-483-5p/HDAC4 axis in diabetic kidney disease.Hormones (Athens). 2025 Mar;24(1):123-135. doi: 10.1007/s42000-024-00606-2. Epub 2024 Oct 8. Hormones (Athens). 2025. PMID: 39375302
-
Research progress on non-coding RNA regulatory networks and targeted therapy in diabetic nephropathy.Front Endocrinol (Lausanne). 2025 Jul 29;16:1625307. doi: 10.3389/fendo.2025.1625307. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40801037 Free PMC article. Review.
-
The potential role of differentially expressed tRNA-derived fragments in high glucose-induced podocytes.Ren Fail. 2024 Dec;46(1):2318413. doi: 10.1080/0886022X.2024.2318413. Epub 2024 Feb 18. Ren Fail. 2024. PMID: 38369750 Free PMC article.
-
Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review.Cell Commun Signal. 2024 Feb 19;22(1):136. doi: 10.1186/s12964-024-01502-3. Cell Commun Signal. 2024. PMID: 38374141 Free PMC article. Review.
-
Role of Semaphorin 3A in Kidney Development and Diseases.Diagnostics (Basel). 2023 Sep 25;13(19):3038. doi: 10.3390/diagnostics13193038. Diagnostics (Basel). 2023. PMID: 37835781 Free PMC article. Review.
References
-
- Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab: TEM 2016; 27: 820–830. - PubMed
-
- Miyauchi M, Toyoda M, Kobayashi K, et al. Hypertrophy and loss of podocytes in diabetic nephropathy. Intern Med 2009; 48: 1615–1620. - PubMed
-
- Lewko B, Stepinski J. Hyperglycemia and mechanical stress: targeting the renal podocyte. J Cell Physiol 2009; 221: 288–295. - PubMed
-
- Fatica A, Bozzoni I. Long non‐coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014; 15: 7–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

