Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes
- PMID: 36583355
- PMCID: PMC9799031
- DOI: 10.1097/HJH.0000000000003330
Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes
Abstract
Objective: Finerenone is a selective nonsteroidal mineralocorticoid receptor antagonist with a short half-life. Its effects on cardiorenal outcomes were thought to be mediated primarily via nonhemodynamic pathways, but office blood pressure (BP) measurements were insufficient to fully assess hemodynamic effects. This analysis assessed the effects of finerenone on 24-h ambulatory BP in patients with chronic kidney disease and type 2 diabetes.
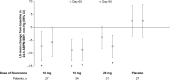
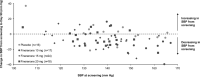
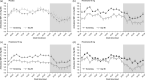
Methods: ARTS-DN (NCT01874431) was a phase 2b trial that randomized 823 patients with type 2 diabetes and chronic kidney disease, with urine albumin-to-creatinine ratio ≥30 mg/g and estimated glomerular filtration rate of 30-90 ml/min per 1.73 m2 to placebo or finerenone (1.25-20 mg once daily in the morning) administered over 90 days. Ambulatory BP monitoring (ABPM) over 24 h was performed in a subset of 240 patients at screening, Day 60, and Day 90.
Results: Placebo-adjusted change in 24-h ABPM systolic BP (SBP) at Day 90 was -8.3 mmHg (95% confidence interval [CI], -16.6 to 0.1) for finerenone 10 mg (n = 27), -11.2 mmHg (95% CI, -18.8 to -3.6) for finerenone 15 mg (n = 34), and -9.9 mmHg (95% CI, -17.7 to -2.0) for finerenone 20 mg (n = 31). Mean daytime and night-time SBP recordings were similarly reduced and finerenone did not increase the incidence of SBP dipping. Finerenone produced a persistent reduction in SBP over the entire 24-h interval.
Conclusions: Finerenone reduced 24-h, daytime, and night-time SBP. Despite a short half-life, changes in BP were persistent over 24 h with once-daily dosing in the morning.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
L.M.R. has received consultancy fees from Bayer. R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc., during the conduct of the study; he also reports personal fees and nonfinancial support from Akebia Therapeutics, Boehringer Ingelheim, Janssen, Relypsa/Vifor Pharma; he has received personal fees from Diamedica and Reata Pharmaceuticals; he is a member of data safety monitoring committees for Chinook and Vertex; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa Inc.; a member of adjudication committees for Bayer; he has served as associate editor of the
Figures

References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018; 71:1269–1324. - PubMed
-
- Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. . Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; 394:1540–1550. - PubMed
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. - PubMed
-
- de Pinho NA, Levin A, Fukagawa M, Hoy WE, Pecoits-Filho R, Reichel H, et al. . Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int 2019; 96:983–994. - PubMed
-
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383:2219–2229. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

