Ultrahigh-Throughput Directed Evolution of a Metal-Free α/β-Hydrolase with a Cys-His-Asp Triad into an Efficient Phosphotriesterase
- PMID: 36583539
- PMCID: PMC9853848
- DOI: 10.1021/jacs.2c10673
Ultrahigh-Throughput Directed Evolution of a Metal-Free α/β-Hydrolase with a Cys-His-Asp Triad into an Efficient Phosphotriesterase
Abstract
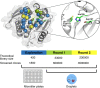
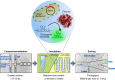
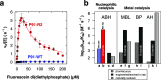
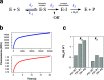
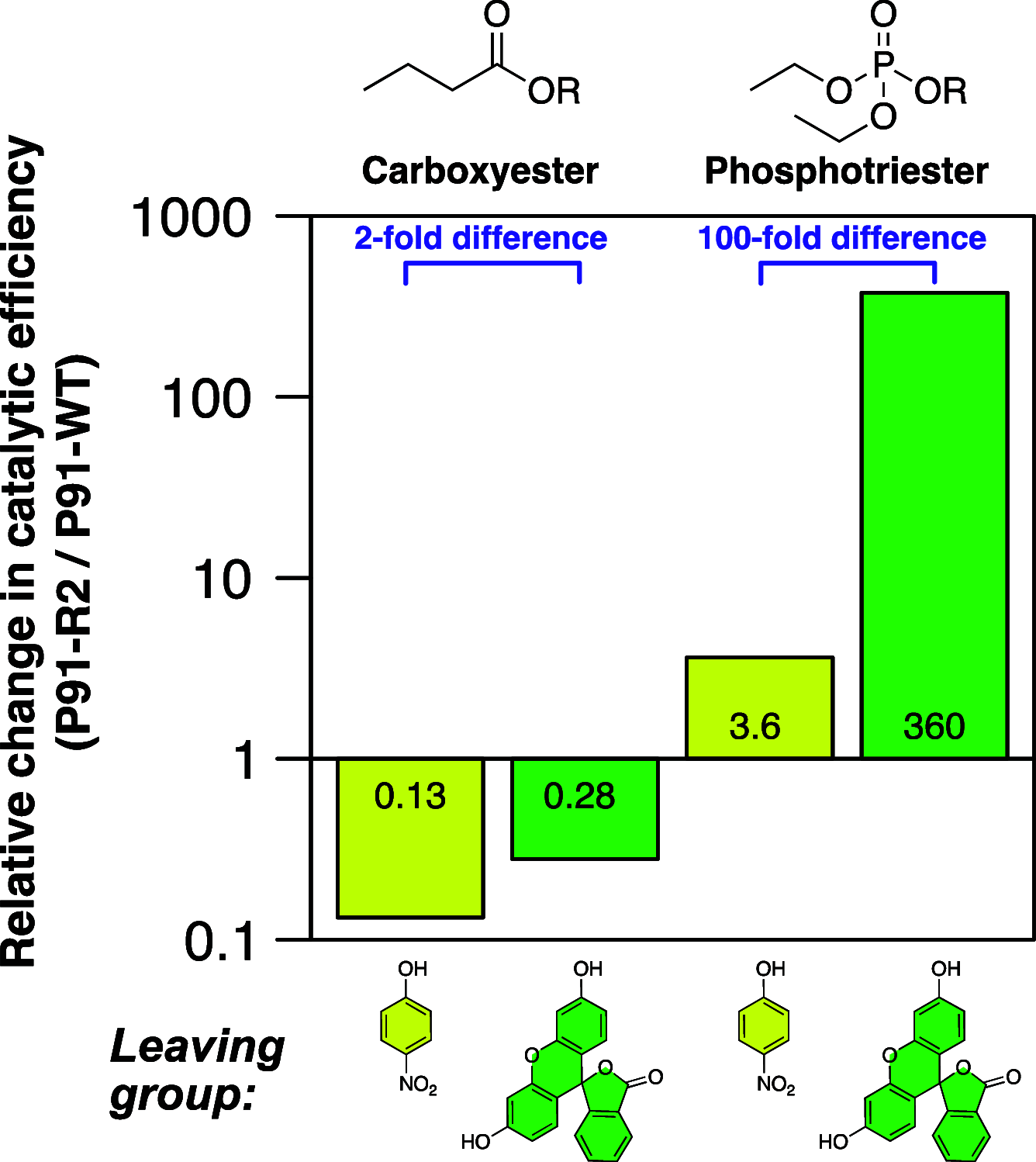
Finding new mechanistic solutions for biocatalytic challenges is key in the evolutionary adaptation of enzymes, as well as in devising new catalysts. The recent release of man-made substances into the environment provides a dynamic testing ground for observing biocatalytic innovation at play. Phosphate triesters, used as pesticides, have only recently been introduced into the environment, where they have no natural counterpart. Enzymes have rapidly evolved to hydrolyze phosphate triesters in response to this challenge, converging onto the same mechanistic solution, which requires bivalent cations as a cofactor for catalysis. In contrast, the previously identified metagenomic promiscuous hydrolase P91, a homologue of acetylcholinesterase, achieves slow phosphotriester hydrolysis mediated by a metal-independent Cys-His-Asp triad. Here, we probe the evolvability of this new catalytic motif by subjecting P91 to directed evolution. By combining a focused library approach with the ultrahigh throughput of droplet microfluidics, we increase P91's activity by a factor of ≈360 (to a kcat/KM of ≈7 × 105 M-1 s-1) in only two rounds of evolution, rivaling the catalytic efficiencies of naturally evolved, metal-dependent phosphotriesterases. Unlike its homologue acetylcholinesterase, P91 does not suffer suicide inhibition; instead, fast dephosphorylation rates make the formation of the covalent adduct rather than its hydrolysis rate-limiting. This step is improved by directed evolution, with intermediate formation accelerated by 2 orders of magnitude. Combining focused, combinatorial libraries with the ultrahigh throughput of droplet microfluidics can be leveraged to identify and enhance mechanistic strategies that have not reached high efficiency in nature, resulting in alternative reagents with novel catalytic machineries.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Catalytic efficiencies of directly evolved phosphotriesterase variants with structurally different organophosphorus compounds in vitro.Arch Toxicol. 2016 Nov;90(11):2711-2724. doi: 10.1007/s00204-015-1626-2. Epub 2015 Nov 26. Arch Toxicol. 2016. PMID: 26612364
-
Interrogation of the Substrate Profile and Catalytic Properties of the Phosphotriesterase from Sphingobium sp. Strain TCM1: An Enzyme Capable of Hydrolyzing Organophosphate Flame Retardants and Plasticizers.Biochemistry. 2015 Dec 29;54(51):7539-49. doi: 10.1021/acs.biochem.5b01144. Epub 2015 Dec 16. Biochemistry. 2015. PMID: 26629649
-
Atropselective Hydrolysis of Chiral Binol-Phosphate Esters Catalyzed by the Phosphotriesterase from Sphingobium sp. TCM1.Biochemistry. 2020 Nov 24;59(46):4463-4469. doi: 10.1021/acs.biochem.0c00831. Epub 2020 Nov 9. Biochemistry. 2020. PMID: 33167613 Free PMC article.
-
Lactonases with organophosphatase activity: structural and evolutionary perspectives.Chem Biol Interact. 2010 Sep 6;187(1-3):370-2. doi: 10.1016/j.cbi.2010.01.039. Epub 2010 Feb 1. Chem Biol Interact. 2010. PMID: 20122908 Review.
-
Detoxification of organophosphate nerve agents by bacterial phosphotriesterase.Toxicol Appl Pharmacol. 2005 Sep 1;207(2 Suppl):459-70. doi: 10.1016/j.taap.2005.02.025. Toxicol Appl Pharmacol. 2005. PMID: 15982683 Review.
Cited by
-
Droplet Microfluidics for High-Throughput Screening and Directed Evolution of Biomolecules.Micromachines (Basel). 2024 Jul 29;15(8):971. doi: 10.3390/mi15080971. Micromachines (Basel). 2024. PMID: 39203623 Free PMC article. Review.
-
Engineering Enzymes for Environmental Sustainability.Angew Chem Weinheim Bergstr Ger. 2023 Dec 21;135(52):e202309305. doi: 10.1002/ange.202309305. Epub 2023 Oct 5. Angew Chem Weinheim Bergstr Ger. 2023. PMID: 38516574 Free PMC article. Review.
-
Ultrahigh Throughput Evolution of Tryptophan Synthase in Droplets via an Aptamer Sensor.ACS Catal. 2024 Apr 10;14(8):6259-6271. doi: 10.1021/acscatal.4c00230. eCollection 2024 Apr 19. ACS Catal. 2024. PMID: 38660603 Free PMC article.
-
Droplet microfluidic screening to engineer angiotensin-converting enzyme 2 (ACE2) catalytic activity.J Biol Eng. 2025 Feb 3;19(1):12. doi: 10.1186/s13036-025-00482-3. J Biol Eng. 2025. PMID: 39901286 Free PMC article.
-
Selection of a promiscuous minimalist cAMP phosphodiesterase from a library of de novo designed proteins.Nat Chem. 2024 Jul;16(7):1200-1208. doi: 10.1038/s41557-024-01490-4. Epub 2024 May 3. Nat Chem. 2024. PMID: 38702405 Free PMC article.
References
-
- Matolcsy G.; Nádasy M.; Andriska V.. Pesticide Chemistry; Elsevier, 1989.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

