Molecular mechanisms and roles of pyroptosis in acute lung injury
- PMID: 36583860
- PMCID: PMC9945565
- DOI: 10.1097/CM9.0000000000002425
Molecular mechanisms and roles of pyroptosis in acute lung injury
Abstract
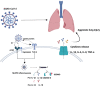
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), which are characterized by excessive inflammation and accompanied by diffuse injury of alveoli, can result in severe respiratory failures. The morbidity and mortality of patients remain high because the major treatments for ALI/ARDS are mainly supportive due to the lack of effective therapies. Numerous studies have demonstrated that the aggravation of coronavirus disease 2019 (COVID-19) leads to severe pneumonia and even ARDS. Pyroptosis, a biological process identified as a type of programed cell death, is mainly triggered by inflammatory caspase activation and is directly meditated by the gasdermin protein family, as well as being associated with the secretion and release of pro-inflammatory cytokines. Clinical and experimental evidence corroborates that pyroptosis of various cells in the lung, such as immune cells and structural cells, may play an important role in the pathogenesis of "cytokine storms" in ALI/ARDS, including those induced by COVID-19. Here, with a focus on ALI/ARDS and COVID-19, we summarized the recent advances in this field and proposed the theory of an inflammatory cascade in pyroptosis to identify new targets and pave the way for new approaches to treat these diseases.
Copyright © 2022 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures


Similar articles
-
Macrophage pyroptosis and its crucial role in ALI/ARDS.Front Immunol. 2025 Feb 14;16:1530849. doi: 10.3389/fimmu.2025.1530849. eCollection 2025. Front Immunol. 2025. PMID: 40028334 Free PMC article. Review.
-
Xuebijing alleviates LPS-induced acute lung injury by downregulating pro-inflammatory cytokine production and inhibiting gasdermin-E-mediated pyroptosis of alveolar epithelial cells.Chin J Nat Med. 2023 Aug;21(8):576-588. doi: 10.1016/S1875-5364(23)60463-7. Chin J Nat Med. 2023. PMID: 37611976
-
Fudosteine attenuates acute lung injury in septic mice by inhibiting pyroptosis via the TXNIP/NLRP3/GSDMD pathway.Eur J Pharmacol. 2022 Jul 5;926:175047. doi: 10.1016/j.ejphar.2022.175047. Epub 2022 May 21. Eur J Pharmacol. 2022. PMID: 35609679
-
Alpha-linolenic acid pretreatment alleviates NETs-induced alveolar macrophage pyroptosis by inhibiting pyrin inflammasome activation in a mouse model of sepsis-induced ALI/ARDS.Front Immunol. 2023 Mar 27;14:1146612. doi: 10.3389/fimmu.2023.1146612. eCollection 2023. Front Immunol. 2023. PMID: 37051243 Free PMC article.
-
MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome.Int J Mol Sci. 2022 May 16;23(10):5545. doi: 10.3390/ijms23105545. Int J Mol Sci. 2022. PMID: 35628354 Free PMC article. Review.
Cited by
-
CircMAPK1 induces cell pyroptosis in sepsis-induced lung injury by mediating KDM2B mRNA decay to epigenetically regulate WNK1.Mol Med. 2024 Sep 19;30(1):155. doi: 10.1186/s10020-024-00932-6. Mol Med. 2024. PMID: 39300342 Free PMC article.
-
Gut microbiota and its metabolic products in acute respiratory distress syndrome.Front Immunol. 2024 Feb 16;15:1330021. doi: 10.3389/fimmu.2024.1330021. eCollection 2024. Front Immunol. 2024. PMID: 38433840 Free PMC article. Review.
-
miRNA-206-3p alleviates LPS-induced acute lung injury via inhibiting inflammation and pyroptosis through modulating TLR4/NF-κB/NLRP3 pathway.Sci Rep. 2024 May 24;14(1):11860. doi: 10.1038/s41598-024-62733-5. Sci Rep. 2024. PMID: 38789583 Free PMC article.
-
USP33 promotes pulmonary microvascular endothelial cell pyroptosis by stabilizing TRAF2 through deubiquitination.Histol Histopathol. 2025 Jul;40(7):1073-1081. doi: 10.14670/HH-18-835. Epub 2024 Oct 17. Histol Histopathol. 2025. PMID: 39506557
-
Mechanisms and Nanomedicine Interventions of Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion: A Mini Review.Int J Nanomedicine. 2025 Jul 25;20:9347-9367. doi: 10.2147/IJN.S533797. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40735744 Free PMC article. Review.
References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967; 2:319–323. doi: 10.1016/s0140-6736(67)90168-7. - PubMed
-
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007; 369:1553–1564. doi: 10.1016/s0140-6736(07)60604-7. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. . Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353:1685–1693. doi: 10.1056/NEJMoa050333. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

