One-year cardiovascular outcomes after coronavirus disease 2019: The cardiovascular COVID-19 registry
- PMID: 36583998
- PMCID: PMC9803130
- DOI: 10.1371/journal.pone.0279333
One-year cardiovascular outcomes after coronavirus disease 2019: The cardiovascular COVID-19 registry
Abstract
Background: The long-term cardiovascular (CV) outcomes of COVID-19 have not been fully explored.
Methods: This was an international, multicenter, retrospective cohort study conducted between February and December 2020. Consecutive patients ≥18 years who underwent a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV2 were included. Patients were classified into two cohorts depending on the nasopharyngeal swab result and clinical status: confirmed COVID-19 (positive RT-PCR) and control (without suggestive symptoms and negative RT-PCR). Data were obtained from electronic records, and clinical follow-up was performed at 1-year. The primary outcome was CV death at 1-year. Secondary outcomes included arterial thrombotic events (ATE), venous thromboembolism (VTE), and serious cardiac arrhythmias. An independent clinical event committee adjudicated events. A Cox proportional hazards model adjusted for all baseline characteristics was used for comparing outcomes between groups. A prespecified landmark analysis was performed to assess events during the post-acute phase (31-365 days).
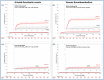
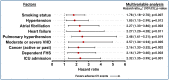
Results: A total of 4,427 patients were included: 3,578 (80.8%) in the COVID-19 and 849 (19.2%) control cohorts. At one year, there were no significant differences in the primary endpoint of CV death between the COVID-19 and control cohorts (1.4% vs. 0.8%; HRadj 1.28 [0.56-2.91]; p = 0.555), but there was a higher risk of all-cause death (17.8% vs. 4.0%; HRadj 2.82 [1.99-4.0]; p = 0.001). COVID-19 cohort had higher rates of ATE (2.5% vs. 0.8%, HRadj 2.26 [1.02-4.99]; p = 0.044), VTE (3.7% vs. 0.4%, HRadj 9.33 [2.93-29.70]; p = 0.001), and serious cardiac arrhythmias (2.5% vs. 0.6%, HRadj 3.37 [1.35-8.46]; p = 0.010). During the post-acute phase, there were no significant differences in CV death (0.6% vs. 0.7%; HRadj 0.67 [0.25-1.80]; p = 0.425), but there was a higher risk of deep vein thrombosis (0.6% vs. 0.0%; p = 0.028). Re-hospitalization rate was lower in the COVID-19 cohort compared to the control cohort (13.9% vs. 20.6%; p = 0.001).
Conclusions: At 1-year, patients with COVID-19 experienced an increased risk of all-cause death and adverse CV events, including ATE, VTE, and serious cardiac arrhythmias, but not CV death.
Study registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT04359927.
Copyright: © 2022 Ortega-Paz et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
GC received research grants from Boston Scientific, Medis, SMT, Siemens, Insight Lifetech. BB reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. HGG reports a relationship with Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Philips, and CorFlow that includes: funding grants. DJA declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi. DJA also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. JRC is consultant for and has received institutional research grants from Edwards Lifesciences, Medtronic and Boston Scientific. MS declares that he is a consultant for Abbott Vascular and iVascular. SB declares that he is a consultant from Boston Scientific, iVascular and Teleflex, and he received speaker’s fee from Abbott Vascular, outside the present work. Other authors have nothing to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ. Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management. Journal of the American Heart Association. 2021;10(3):e019650. Epub 2020/11/25. doi: 10.1161/JAHA.120.019650 . - DOI - PMC - PubMed
-
- Ortega-Paz L, Galli M, Capodanno D, Franchi F, Rollini F, Bikdeli B, et al.. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021. Epub 2021/09/15. doi: 10.1093/ehjcvp/pvab070 . - DOI - PMC - PubMed
-
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al.. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75(18):2352–71. Epub 20200319. doi: 10.1016/j.jacc.2020.03.031 . - DOI - PMC - PubMed
-
- Ortega-Paz L, Galli M, Angiolillo DJ. Updated meta-analysis of randomized controlled trials on the safety and efficacy of different prophylactic anticoagulation dosing regimens in non-critically ill hospitalized patients with COVID-19. Eur Heart J Cardiovasc Pharmacother. 2022;8(3):E15–E7. Epub 2022/02/03. doi: 10.1093/ehjcvp/pvac010 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

