Association between ustekinumab therapy and changes in specific anti-microbial response, serum biomarkers, and microbiota composition in patients with IBD: A pilot study
- PMID: 36584073
- PMCID: PMC9803183
- DOI: 10.1371/journal.pone.0277576
Association between ustekinumab therapy and changes in specific anti-microbial response, serum biomarkers, and microbiota composition in patients with IBD: A pilot study
Abstract
Background: Ustekinumab, is a new therapy for patients with IBD, especially for patients suffering from Crohn's disease (CD) who did not respond to anti-TNF treatment. To shed light on the longitudinal effect of ustekinumab on the immune system, we investigated the effect on skin and gut microbiota composition, specific immune response to commensals, and various serum biomarkers.
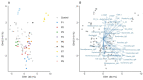
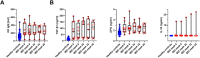
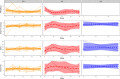
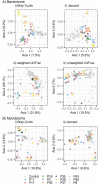
Methodology/principal findings: We recruited 11 patients with IBD who were monitored over 40 weeks of ustekinumab therapy and 39 healthy controls (HC). We found differences in the concentrations of serum levels of osteoprotegerin, TGF-β1, IL-33, and serum IgM antibodies against Lactobacillus plantarum between patients with IBD and HC. The levels of these biomarkers did not change in response to ustekinumab treatment or with disease improvement during the 40 weeks of observation. Additionally, we identified differences in stool abundance of uncultured Subdoligranulum, Faecalibacterium, and Bacteroides between patients with IBD and HC.
Conclusion/significance: In this preliminary study, we provide a unique overview of the longitudinal monitoring of fecal and skin microbial profiles as well as various serum biomarkers and humoral and cellular response to gut commensals in a small cohort of patients with IBD on ustekinumab therapy.
Copyright: © 2022 Rob et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

