Mechanical strain induces ex vivo expansion of periosteum
- PMID: 36584151
- PMCID: PMC9803115
- DOI: 10.1371/journal.pone.0279519
Mechanical strain induces ex vivo expansion of periosteum
Abstract
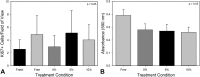
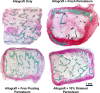
Segmental bone defects present complex clinical challenges. Nonunion, malunion, and infection are common sequalae of autogenous bone grafts, allografts, and synthetic bone implants due to poor incorporation with the patient's bone. The current project explores the osteogenic properties of periosteum to facilitate graft incorporation. As tissue area is a natural limitation of autografting, mechanical strain was implemented to expand the periosteum. Freshly harvested, porcine periosteum was strained at 5 and 10% per day for 10 days with non-strained and free-floating samples serving as controls. Total tissue size, viability and histologic examination revealed that strain increased area to a maximum of 1.6-fold in the 10% daily strain. No change in tissue anatomy or viability via MTT or Ki67 staining and quantification was observed among groups. The osteogenic potential of the mechanical expanded periosteum was then examined in vivo. Human cancellous allografts were wrapped with 10% per day strained, fresh, free-floating, or no porcine periosteum and implanted subcutaneously into female, athymic mice. Tissue was collected at 8- and 16-weeks. Gene expression analysis revealed a significant increase in alkaline phosphatase and osteocalcin in the fresh periosteum group at 8-weeks post implantation compared to all other groups. Values among all groups were similar at week 16. Additionally, histological assessment with H&E and Masson-Goldner Trichrome staining showed that all periosteal groups outperformed the non-periosteal allograft, with fresh periosteum demonstrating the highest levels of new tissue mineralization at the periosteum-bone interface. Overall, mechanical expansion of the periosteum can provide increased area for segmental healing via autograft strategies, though further studies are needed to explore culture methodology to optimize osteogenic potential.
Copyright: © 2022 Walker et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48(1): 1–11. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

