Addressing the quality challenge of a human biospecimen biobank through the creation of a quality management system
- PMID: 36584180
- PMCID: PMC9803146
- DOI: 10.1371/journal.pone.0278780
Addressing the quality challenge of a human biospecimen biobank through the creation of a quality management system
Abstract
Background: The objective of the COMET (COllection of MEtabolic Tissues) biobank project is to create a high-quality collection of insulin-sensitive tissues (liver, muscle, adipose tissues, and epiploic artery) and blood sample derivatives (plasma, serum, DNA and RNA), collected from 270 grade 2-3 obese patients undergoing bariatric surgery. Relevant data on patient such as clinical/biological characteristics and sample handling are also collected. For this, our aim was to establish a Quality Management System (QMS) to meet the reliability and quality requirements necessary for its scientific exploitation.
Materials and methods: The COMET QMS includes: (1) Quality Assurance to standardize all stages of the biobanking process, (2) Quality Controls on samples from the first patients included in order to validate the sample management process and ensure reproducible quality; and 3) "in process" Quality Controls to ensure the reliability of the storage procedures and the stability of the samples over time.
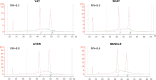
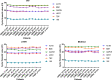
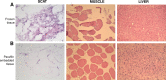
Results: For serum and plasma, several corrective actions, such as temperature handling and centrifugation conditions, were made to the protocol and led to improvement of the volume and quality of samples. Regarding DNA, all samples evaluated achieved a satisfactory level of purity and integrity and most of them yielded the required DNA quantity. All frozen tissue samples had RNAs of good purity. RNA quality was confirmed by RIN, achieving values in most cases over 7 and efficient amplification of housekeeping genes by RT-qPCR, with no significant differences among samples from the same tissue type. In the "in process" Quality Controls, DNA, RNA, and histological integrity of tissues showed no differences among samples after different preservation times.
Conclusion: Quality Control results have made it possible to validate the entire biobank process and confirm the utility of implementing QMS to guarantee the quality of a biospecimen collection.
Copyright: © 2022 Servais et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- COllection Of Metabolic Tissues (COMET). http://cometbiobank.com. Accessed 8 June 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

