Prostaglandin F2α drives female pheromone signaling in cichlids, revealing a basis for evolutionary divergence in olfactory signaling
- PMID: 36584295
- PMCID: PMC9910499
- DOI: 10.1073/pnas.2214418120
Prostaglandin F2α drives female pheromone signaling in cichlids, revealing a basis for evolutionary divergence in olfactory signaling
Abstract
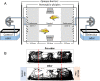
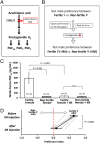
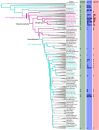
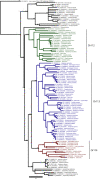
Pheromones play essential roles in reproduction in many species. Prostaglandin F2α (PGF2α) acts as a female reproductive hormone and as a sex pheromone in some species. An olfactory receptor (OR) for PGF2α was recently discovered in zebrafish, but this signaling pathway is evolutionarily labile. To understand the evolution of signals that attract males to fertile females, we used the African cichlid Astatotilapia burtoni and found that adult males strongly prefer fertile female odors. Injection of a prostaglandin synthesis inhibitor abolishes this attractivity of fertile females, indicating these hormones are necessary for pheromonal signaling. Unlike zebrafish, A. burtoni males are insensitive to PGF2α, but they do exhibit strong preference for females injected with PGF2α. This attractiveness is independent of the PGF2α hormonal receptor Ptgfr, indicating that this pheromone signaling derives from PGF2α metabolization into a yet-undiscovered pheromone. We further discovered that fish that are insensitive to PGF2α lack an ortholog for the OR Or114 that zebrafish use to detect PGF2α. These results indicate that PGF2α itself does not directly induce male preference in cichlids. Rather, it plays a vital role that primes females to become attractive via an alternative male OR.
Keywords: cichlid; hormone; olfactory receptor; pheromone; prostaglandin.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
A pheromone receptor in cichlid fish mediates attraction to females but inhibits male parental care.Curr Biol. 2024 Sep 9;34(17):3866-3880.e7. doi: 10.1016/j.cub.2024.07.029. Epub 2024 Aug 1. Curr Biol. 2024. PMID: 39094572
-
A Neural Basis for Control of Cichlid Female Reproductive Behavior by Prostaglandin F2α.Curr Biol. 2016 Apr 4;26(7):943-9. doi: 10.1016/j.cub.2016.01.067. Epub 2016 Mar 17. Curr Biol. 2016. PMID: 26996507 Free PMC article.
-
Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior.Nat Neurosci. 2016 Jul;19(7):897-904. doi: 10.1038/nn.4314. Epub 2016 May 30. Nat Neurosci. 2016. PMID: 27239939
-
Pheromone reception in moths: from molecules to behaviors.Prog Mol Biol Transl Sci. 2015;130:109-28. doi: 10.1016/bs.pmbts.2014.11.005. Epub 2014 Dec 16. Prog Mol Biol Transl Sci. 2015. PMID: 25623339 Review.
-
Hormonally derived sex pheromones in fish: exogenous cues and signals from gonad to brain.Can J Physiol Pharmacol. 2003 Apr;81(4):329-41. doi: 10.1139/y03-024. Can J Physiol Pharmacol. 2003. PMID: 12769225 Review.
Cited by
-
Olfactory GnRH3 crypt sensory neurons transduce sex pheromone signals to induce male courtship behavior in zebrafish.Sci China Life Sci. 2025 Aug;68(8):2191-2205. doi: 10.1007/s11427-025-2917-5. Epub 2025 May 8. Sci China Life Sci. 2025. PMID: 40347216
-
A pheromone receptor in cichlid fish mediates attraction to females but inhibits male parental care.Curr Biol. 2024 Sep 9;34(17):3866-3880.e7. doi: 10.1016/j.cub.2024.07.029. Epub 2024 Aug 1. Curr Biol. 2024. PMID: 39094572
-
Pheromone Perception in Fish: Mechanisms and Modulation by Internal Status.Integr Comp Biol. 2023 Aug 23;63(2):407-427. doi: 10.1093/icb/icad049. Integr Comp Biol. 2023. PMID: 37263784 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

