Engineering an inhibitor-resistant human CSF1R variant for microglia replacement
- PMID: 36584406
- PMCID: PMC9814156
- DOI: 10.1084/jem.20220857
Engineering an inhibitor-resistant human CSF1R variant for microglia replacement
Abstract
Hematopoietic stem cell transplantation (HSCT) can replace endogenous microglia with circulation-derived macrophages but has high mortality. To mitigate the risks of HSCT and expand the potential for microglia replacement, we engineered an inhibitor-resistant CSF1R that enables robust microglia replacement. A glycine to alanine substitution at position 795 of human CSF1R (G795A) confers resistance to multiple CSF1R inhibitors, including PLX3397 and PLX5622. Biochemical and cell-based assays show no discernable gain or loss of function. G795A- but not wildtype-CSF1R expressing macrophages efficiently engraft the brain of PLX3397-treated mice and persist after cessation of inhibitor treatment. To gauge translational potential, we CRISPR engineered human-induced pluripotent stem cell-derived microglia (iMG) to express G795A. Xenotransplantation studies demonstrate that G795A-iMG exhibit nearly identical gene expression to wildtype iMG, respond to inflammatory stimuli, and progressively expand in the presence of PLX3397, replacing endogenous microglia to fully occupy the brain. In sum, we engineered a human CSF1R variant that enables nontoxic, cell type, and tissue-specific replacement of microglia.
© 2022 Chadarevian et al.
Conflict of interest statement
Disclosures: M.L. Bennett and F.C. Bennett are co-inventors on a pending patent filed by The Board of Trustees of The Leland Stanford Junior University (application 16/566,675) related to methods of microglia replacement. M. Blurton-Jones, J.P. Chadarevian, H. Davtyan, J. Hasselmann, W. England, and R.C. Spitale are co-inventors on a pending patent filed by the University of California Regents (application 63/169,578) related to genetic modification of cells to confer resistance to CSF1R antagonists. M. Blurton-Jones is a co-inventor of patent WO/2018/160496, related to the differentiation of human pluripotent stem cells into microglia. F.C. Bennett is a consultant for and shareholder in Glia Biotherapeutics Inc. M. Blurton-Jones and R.C. Spitale are co-founders of NovoGlia Inc. No other disclosures were reported.
Figures



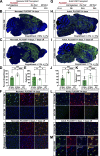

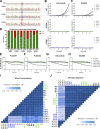
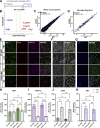
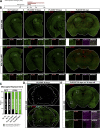
Comment in
-
Engineering mighty microglia.J Exp Med. 2023 Mar 6;220(3):e20222070. doi: 10.1084/jem.20222070. Epub 2023 Jan 17. J Exp Med. 2023. PMID: 36648421 Free PMC article.
References
-
- Andrews, S. 2014. FastQC a quality-control tool for high-throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Publication types
MeSH terms
Substances
Grants and funding
- R43 NS125730/NS/NINDS NIH HHS/United States
- S10 OD021718/OD/NIH HHS/United States
- T32 AG073088/AG/NIA NIH HHS/United States
- S10 RR025496/RR/NCRR NIH HHS/United States
- T32 MH014654/MH/NIMH NIH HHS/United States
- R01 AG061895/AG/NIA NIH HHS/United States
- S10 OD010794/OD/NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- T32 GM008076/GM/NIGMS NIH HHS/United States
- R01 NS120960/NS/NINDS NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- T32 GM007170/GM/NIGMS NIH HHS/United States
- T32 NS007413/NS/NINDS NIH HHS/United States
- K12 GM081259/GM/NIGMS NIH HHS/United States
- RF1 DA048813/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

