Three-dimensional quantification of fibrosis and ossification after cochlear implantation via virtual re-sectioning: Potential implications for residual hearing
- PMID: 36584546
- PMCID: PMC10942756
- DOI: 10.1016/j.heares.2022.108681
Three-dimensional quantification of fibrosis and ossification after cochlear implantation via virtual re-sectioning: Potential implications for residual hearing
Abstract
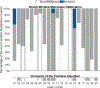
Hearing preservation may be achieved initially in the majority of patients after cochlear implantation, however, a significant proportion of these patients experience delayed hearing loss months or years later. A prior histological report in a case of delayed hearing loss suggested a potential cochlear mechanical origin of this hearing loss due to tissue fibrosis, and older case series highlight the frequent findings of post-implantation fibrosis and neoosteogenesis though without a focus on the impact on residual hearing. Here we present the largest series (N = 20) of 3-dimensionally reconstructed cochleae based on digitally scanned histologic sections from patients who were implanted during their lifetime. All patients were implanted with multichannel electrodes via a cochleostomy or an extended round window insertion. A quantified analysis of intracochlear tissue formation was carried out via virtual re-sectioning orthogonal to the cochlear spiral. Intracochlear tissue formation was present in every case. On average 33% (SD 14%) of the total cochlear volume was occupied by new tissue formation, consisting of 26% (SD 12%) fibrous and 7% (SD 6%) bony tissue. The round window was completely covered by fibro-osseous tissue in 85% of cases and was associated with an obstruction of the cochlear aqueduct in 100%. The basal part of the basilar membrane was at least partially abutted by the electrode or new tissue formation in every case, while the apical region, corresponding with a characteristic frequency of < 500 Hz, appeared normal in 89%. This quantitative analysis shows that after cochlear implantation via extended round window or cochleostomy, intracochlear fibrosis and neoossification are present in all cases at anatomical locations that could impact normal inner ear mechanics.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest None
Figures

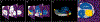

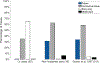

References
-
- Banakis Hartl RM, Mattingly JK, Greene NT, Jenkins HA, Cass SP, Tollin DJ, 2016. A Preliminary Investigation of the Air-Bone Gap: Changes in Intracochlear Sound Pressure With Air- and Bone-conducted Stimuli After Cochlear Implantation. Otol. Neurotol. 37, 1291–1299. 10.1097/MAO.0000000000001184 - DOI - PMC - PubMed
-
- Choong JK, Hampson AJ, Brody KM, Lo J, Bester CW, Gummer AW, Reynolds NP, O’Leary SJ, 2020. Nanomechanical mapping reveals localized stiffening of the basilar membrane after cochlear implantation: Basilar membrane stiffness after CI. Hear. Res. 385, 107846. 10.1016/j.heares.2019.107846 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

