KATs off: Biomedical insights from lysine acetyltransferase inhibitors
- PMID: 36584580
- PMCID: PMC9870960
- DOI: 10.1016/j.cbpa.2022.102255
KATs off: Biomedical insights from lysine acetyltransferase inhibitors
Abstract
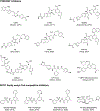
Lysine acetyltransferase (KAT) enzymes including the p300, MYST, and GCN5 families play major roles in modulating the structure of chromatin and regulating transcription. Because of their dysregulation in various disease states including cancer, efforts to develop inhibitors of KATs have steadily gained momentum. Here we provide an overview of recent progress on the development of high quality chemical probes of the p300 and MYST family of KATs and how they are emerging as useful tools for basic and translational investigation.
Keywords: Acetyltransferase; CBP; GCN5; MYST; PCAF; p300.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Philip Cole reports financial support was provided by National Institute of General Medical Sciences. Philip Cole reports a relationship with Acylin Therapeutics and Abbvie Inc that includes: consulting or advisory and equity or stocks. Philip Cole has patent #U.S. patent# 9,005,670 issued April 14, 2015 with royalties paid to Acylin Therapeutics. Co-editor for COCB.
Figures
Similar articles
-
KAT tales: Functions of Gcn5 and PCAF lysine acetyltransferases in SAGA and ATAC.J Biol Chem. 2024 Oct;300(10):107744. doi: 10.1016/j.jbc.2024.107744. Epub 2024 Sep 1. J Biol Chem. 2024. PMID: 39222683 Free PMC article. Review.
-
Genome-scale analysis of regulatory protein acetylation enzymes from photosynthetic eukaryotes.BMC Genomics. 2017 Jul 5;18(1):514. doi: 10.1186/s12864-017-3894-0. BMC Genomics. 2017. PMID: 28679357 Free PMC article.
-
Profiling Cellular Substrates of Lysine Acetyltransferases GCN5 and p300 with Orthogonal Labeling and Click Chemistry.ACS Chem Biol. 2017 Jun 16;12(6):1547-1555. doi: 10.1021/acschembio.7b00114. Epub 2017 Apr 26. ACS Chem Biol. 2017. PMID: 28426192
-
Identification of Novel Protein Lysine Acetyltransferases in Escherichia coli.mBio. 2018 Oct 23;9(5):e01905-18. doi: 10.1128/mBio.01905-18. mBio. 2018. PMID: 30352934 Free PMC article.
-
Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer.Front Endocrinol (Lausanne). 2022 Aug 17;13:886594. doi: 10.3389/fendo.2022.886594. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060957 Free PMC article. Review.
Cited by
-
Chemically induced degradation of epigenetic targets.Chem Soc Rev. 2023 Jul 3;52(13):4313-4342. doi: 10.1039/d3cs00100h. Chem Soc Rev. 2023. PMID: 37314393 Free PMC article. Review.
-
Current trends in clinical trials and the development of small molecule epigenetic inhibitors as cancer therapeutics.Epigenomics. 2024 Apr 19;16(9):671-80. doi: 10.2217/epi-2023-0443. Online ahead of print. Epigenomics. 2024. PMID: 38639711 Free PMC article. Review.
-
Structural and functional characterization of CREB-binding protein (CREBBP) as a histone propionyltransferase.J Biol Chem. 2025 Jul 2;301(8):110444. doi: 10.1016/j.jbc.2025.110444. Online ahead of print. J Biol Chem. 2025. PMID: 40615044 Free PMC article.
-
Development of p300-targeting degraders with enhanced selectivity and onset of degradation.RSC Med Chem. 2025 Mar 3;16(5):2049-2060. doi: 10.1039/d4md00969j. eCollection 2025 May 22. RSC Med Chem. 2025. PMID: 40093518 Free PMC article.
-
The role of acetylation in obesity-induced cardiac metabolic alterations.J Pharm Pharm Sci. 2024 Jul 23;27:13080. doi: 10.3389/jpps.2024.13080. eCollection 2024. J Pharm Pharm Sci. 2024. PMID: 39109269 Free PMC article. Review.
References
-
- Li X; Liu S; Li X; Li XD YEATS Domains as Novel Epigenetic Readers: Structures, Functions, and Inhibitor Development. ACS Chem Biol. 2021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

