Development of potent and selective degraders of PI5P4Kγ
- PMID: 36584631
- PMCID: PMC10150581
- DOI: 10.1016/j.ejmech.2022.115027
Development of potent and selective degraders of PI5P4Kγ
Abstract
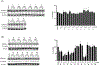
Phosphatidylinositol 5-phosphate 4-kinases (PI5P4Ks), a family of three members in mammals (α, β and γ), have emerged as potential therapeutic targets due to their role in regulating many important cellular signaling pathways. In comparison to the PI5P4Kα and PI5P4Kβ, which usually have similar expression profiles across cancer cells, PI5P4Kγ exhibits distinct expression patterns, and pathological functions for PI5P4Kγ have been proposed in the context of cancer and neurodegenerative diseases. PI5P4Kγ has very low kinase activity and has been proposed to inhibit the PI4P5Ks through scaffolding function, providing a rationale for developing a selective PI5P4Kγ degrader. Here, we report the development and characterization of JWZ-1-80, a first-in-class PI5P4Kγ degrader. JWZ-1-80 potently degrades PI5P4Kγ via the ubiquitin-proteasome system and exhibits proteome-wide selectivity and is therefore a useful tool compound for further dissecting the biological functions of PI5P4Kγ.
Keywords: PI5P4Kγ; PROTAC; Protein degrader; Selective.
Copyright © 2022 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: PI5P4Kγ degraders developed in this manuscript are licensed to Larkspur where N.S.G. and L.C.C. have a financial interest. W.J., E.S.W., T.D.M., K.A.D., E.S.F., T.Z., L.C.C. and N.S.G are inventors on PI5P4Kγ patent (WO 2022140554A1). N.S.G. is a founder, science advisory board member (SAB) and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Larkspur (board member) and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline and Sanofi. T.Z. is a scientific funder, equity holder and consultant of Matchpoint. L.C.C. is a founder and scientific advisory board member of Agios Pharmaceuticals, Faeth Therapeutics, Petra Pharma Corporation, Larkspur Therapeutics and Volastra Pharmaceuticals, and scientific advisory board member for Scorpion Therapeutics. E.S.F. is a founder, scientific advisory board (SAB) member, and equity holder of Civetta Therapeutics, Lighthorse Therapeutics, Proximity Therapeutics, and Neomorph, Inc. (board member). E.S.F. is an equity holder and SAB member for Avilar Therapeutics and Photys Therapeutics and a consultant to Novartis, Sanofi, EcoR1 Capital, and Deerfield. The Fischer lab receives or has received research funding from Novartis, Ajax, and Astellas. K.A.D. is a consultant to Kronos Bio and Neomorph Inc.
Figures


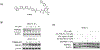

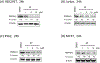


Similar articles
-
The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P-binding site.Biochem J. 2015 Mar 1;466(2):359-67. doi: 10.1042/BJ20141333. Biochem J. 2015. PMID: 25495341 Free PMC article.
-
Development of Selective Phosphatidylinositol 5-Phosphate 4-Kinase γ Inhibitors with a Non-ATP-competitive, Allosteric Binding Mode.J Med Chem. 2022 Feb 24;65(4):3359-3370. doi: 10.1021/acs.jmedchem.1c01819. Epub 2022 Feb 11. J Med Chem. 2022. PMID: 35148092 Free PMC article.
-
The Identification of Potent, Selective, and Brain Penetrant PI5P4Kγ Inhibitors as In Vivo-Ready Tool Molecules.J Med Chem. 2023 Jan 12;66(1):804-821. doi: 10.1021/acs.jmedchem.2c01693. Epub 2022 Dec 14. J Med Chem. 2023. PMID: 36516442 Free PMC article.
-
Phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ), a lipid signalling enigma.Adv Biol Regul. 2016 May;61:47-50. doi: 10.1016/j.jbior.2015.11.007. Epub 2015 Dec 2. Adv Biol Regul. 2016. PMID: 26710750 Review.
-
Targeted protein degrader development for cancer: advances, challenges, and opportunities.Trends Pharmacol Sci. 2023 May;44(5):303-317. doi: 10.1016/j.tips.2023.03.003. Trends Pharmacol Sci. 2023. PMID: 37059054 Review.
Cited by
-
The Genetic Background of Abnormalities in Metabolic Pathways of Phosphoinositides and Their Linkage with the Myotubular Myopathies, Neurodegenerative Disorders, and Carcinogenesis.Biomolecules. 2023 Oct 19;13(10):1550. doi: 10.3390/biom13101550. Biomolecules. 2023. PMID: 37892232 Free PMC article. Review.
-
PI5P4K inhibitors: promising opportunities and challenges.FEBS J. 2025 Sep;292(17):4446-4476. doi: 10.1111/febs.17393. Epub 2025 Jan 19. FEBS J. 2025. PMID: 39828902 Free PMC article. Review.
-
Phosphoinositide kinases in cancer: from molecular mechanisms to therapeutic opportunities.Nat Rev Cancer. 2025 Jun;25(6):463-487. doi: 10.1038/s41568-025-00810-1. Epub 2025 Apr 3. Nat Rev Cancer. 2025. PMID: 40181165 Review.
-
Targeting phosphoinositide signaling in cancer: relevant techniques to study lipids and novel avenues for therapeutic intervention.Front Cell Dev Biol. 2023 Oct 25;11:1297355. doi: 10.3389/fcell.2023.1297355. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37954209 Free PMC article.
-
PROTACs: A novel strategy for cancer drug discovery and development.MedComm (2020). 2023 May 29;4(3):e290. doi: 10.1002/mco2.290. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37261210 Free PMC article. Review.
References
-
- Rameh LE, Tolias KF, Duckworth BC, Cantley LC, A new pathway for synthesis of phosphatidylinositol-4, 5-bisphosphate, Nature 390 (6656) (1997) 192–196. - PubMed
-
- Rameh LE, Cantley LC, The role of phosphoinositide 3-kinase lipid products in cell function, J. Biol. Chem 274 (13) (1999) 8347–8350. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

