Genetic and pharmacological modulation of DNA mismatch repair heterogeneous tumors promotes immune surveillance
- PMID: 36584674
- PMCID: PMC9833846
- DOI: 10.1016/j.ccell.2022.12.003
Genetic and pharmacological modulation of DNA mismatch repair heterogeneous tumors promotes immune surveillance
Abstract
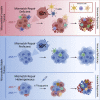
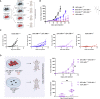
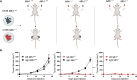
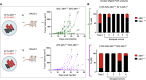
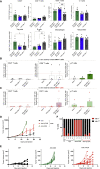
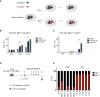
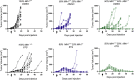
Patients affected by colorectal cancer (CRC) with DNA mismatch repair deficiency (MMRd), often respond to immune checkpoint blockade therapies, while those with mismatch repair-proficient (MMRp) tumors generally do not. Interestingly, a subset of MMRp CRCs contains variable fractions of MMRd cells, but it is unknown how their presence impacts immune surveillance. We asked whether modulation of the MMRd fraction in MMR heterogeneous tumors acts as an endogenous cancer vaccine by promoting immune surveillance. To test this hypothesis, we use isogenic MMRp (Mlh1+/+) and MMRd (Mlh1-/-) mouse CRC cells. MMRp/MMRd cells mixed at different ratios are injected in immunocompetent mice and tumor rejection is observed when at least 50% of cells are MMRd. To enrich the MMRd fraction, MMRp/MMRd tumors are treated with 6-thioguanine, which leads to tumor rejection. These results suggest that genetic and pharmacological modulation of the DNA mismatch repair machinery potentiate the immunogenicity of MMR heterogeneous tumors.
Keywords: 6-thioguanine; heterogeneity; immune checkpoint blockade; immune evasion; immune surveillance; microsatellite unstable tumors (MSI); mismatch repair; temozolomide.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.Bardelli served in a consulting/advisory role for Illumina, Inivata and Guardant Health. The transfer of certain materials to third parties is subject to terms contained within license and intellectual property agreements held between NeoPhore, the University of Turin, A.Bardelli and G.G. A.Bardelli and G.G. are cofounders and shareholders of NeoPhore limited. S.A. is a cofounder and shareholder of CheckMab SRL. A.Bardelli is a member of the scientific advisory board of Neophore, Inivata, and Roche Genentech CRC Advisory Board. A. Bardelli reports grants/research support from Neophore, AstraZeneca, and Inivata. C.M. reports personal consultancy fees from Bayer, Roche and Daichii Sankyo-AstraZeneca outside the scope of the present work. G.M. received honoraria from COR2ED outside the scope of the present work. The remaining authors declares no conflict of interest.
Figures


Comment in
-
Harnessing the therapeutic vulnerability of MMR heterogeneity in colorectal cancer.Cell Rep Med. 2023 Jan 17;4(1):100908. doi: 10.1016/j.xcrm.2022.100908. Cell Rep Med. 2023. PMID: 36652917 Free PMC article.
References
-
- Germano G., Amirouchene-Angelozzi N., Rospo G., Bardelli A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 2018;8:1518–1528. doi: 10.1158/2159-8290.CD-18-0150. - DOI - PubMed
-
- Turajlic S., Litchfield K., Xu H., Rosenthal R., McGranahan N., Reading J.L., Wong Y.N.S., Rowan A., Kanu N., Al Bakir M., et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

