Updating memories of unwanted emotions during human sleep
- PMID: 36584677
- PMCID: PMC9979073
- DOI: 10.1016/j.cub.2022.12.004
Updating memories of unwanted emotions during human sleep
Abstract
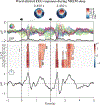
Post-learning sleep contributes to memory consolidation. Yet it remains contentious whether sleep affords opportunities to modify or update emotional memories, particularly when people would prefer to forget those memories. Here, we attempted to update memories during sleep, using spoken positive words paired with cues to recent memories of aversive events. Affective updating using positive words during human non-rapid eye movement (NREM) sleep, compared with using neutral words instead, reduced negative affective judgments in post-sleep tests, suggesting that the recalled events were perceived as less aversive. Electroencephalogram (EEG) analyses showed that positive words modulated theta and spindle/sigma activity; specifically, to the extent that theta power was larger for the positive words than for the memory cues that followed, participants judged the memory cues less negatively. Moreover, to the extent that sigma power was larger for the positive words than for the memory cues that followed, participants forgot more episodic details about aversive events. Notably, when the onset of individual positive words coincided with the up-phase of slow oscillations (a state characterized by increased cortical excitability during NREM sleep), affective updating was more successful. In sum, we altered the affective content of memories via the strategic pairing of positive words and memory cues during sleep, linked with EEG theta power increases and the slow oscillation up-phase. These findings suggest novel possibilities for modifying unwanted memories during sleep, which would not require people to consciously confront memories that they prefer to avoid.
Keywords: memory editing; sleep learning; sleep spindle; slow oscillation; targeted memory reactivation; theta power.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Talamini LM, and Juan E (2020). Sleep as a window to treat affective disorders. Curr. Opin. Behav. Sci 33, 99–108. 10.1016/j.cobeha.2020.02.002. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

