Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation
- PMID: 36584817
- PMCID: PMC9971054
- DOI: 10.1016/j.jcmgh.2022.12.012
Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation
Abstract
Background and aims: The intestinal mucosa undergoes a continual process of proliferation, differentiation, and apoptosis. An imbalance in this highly regimented process within the intestinal crypts is associated with several intestinal pathologies. Although metabolic changes are known to play a pivotal role in cell proliferation and differentiation, how glycolysis contributes to intestinal epithelial homeostasis remains to be defined.
Methods: Small intestines were harvested from mice with specific hexokinase 2 (HK2) deletion in the intestinal epithelium or LGR5+ stem cells. Glycolysis was measured using the Seahorse XFe96 analyzer. Expression of phospho-p38 mitogen-activated protein kinase, the transcription factor atonal homolog 1, and intestinal cell differentiation markers lysozyme, mucin 2, and chromogranin A were determined by Western blot, quantitative real-time reverse transcription polymerase chain reaction, or immunofluorescence, and immunohistochemistry staining.
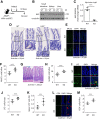
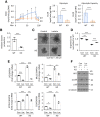
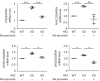
Results: HK2 is a target gene of Wnt signaling in intestinal epithelium. HK2 knockout or inhibition of glycolysis resulted in increased numbers of Paneth, goblet, and enteroendocrine cells and decreased intestinal stem cell self-renewal. Mechanistically, HK2 knockout resulted in activation of p38 mitogen-activated protein kinase and increased expression of ATOH1; inhibition of p38 mitogen-activated protein kinase signaling attenuated the phenotypes induced by HK2 knockout in intestinal organoids. HK2 knockout significantly decreased glycolysis and lactate production in intestinal organoids; supplementation of lactate or pyruvate reversed the phenotypes induced by HK2 knockout.
Conclusions: Our results show that HK2 regulates intestinal stem cell self-renewal and differentiation through p38 mitogen-activated protein kinase/atonal homolog 1 signaling pathway. Our findings demonstrate an essential role for glycolysis in maintenance of intestinal stem cell function.
Keywords: Glycolysis; HK2; Intestinal Stem Cells; Metabolism.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Gersemann M., Wehkamp J., Stange E.F. Innate immune dysfunction in inflammatory bowel disease. J Intern Med. 2012;271:421–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

