Acute exposure to low-dose bisphenol A delays cardiac repolarization in female canine heart - Implication for proarrhythmic toxicity in large animals
- PMID: 36584932
- PMCID: PMC9852101
- DOI: 10.1016/j.fct.2022.113589
Acute exposure to low-dose bisphenol A delays cardiac repolarization in female canine heart - Implication for proarrhythmic toxicity in large animals
Abstract
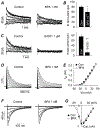
Bisphenol A (BPA) is a common environmental chemical with a range of potential adverse health effects. The impact of environmentally-relevant low dose of BPA on the electrical properties of the hearts of large animals (e.g., dog, human) is poorly defined. Perturbation of cardiac electrical properties is a key arrhythmogenic mechanism. In particular, delay of ventricular repolarization and prolongation of the QT interval of the electrocardiogram is a marker for the risk of malignant arrhythmias. We examined the acute effect of 10-9 M BPA on the electrical properties of female canine ventricular myocytes and tissues. BPA rapidly delayed action potential repolarization and prolonged action potential duration (APD). The dose response curve of BPA on APD was nonmonotonic. BPA rapidly inhibited the IKr K+ current and ICaL Ca2+ current. Computational modeling indicated that the effect of BPA on APD can be accounted for by its suppression of IKr. At the tissue level, BPA acutely prolonged the QT interval in 4 left ventricular wedges. ERβ signaling contributed to the acute effects of BPA on ventricular repolarization. Our results demonstrate that BPA has QT prolongation liability in female canine hearts. These findings have implication for the potential proarrhythmic cardiac toxicity of BPA in large animals.
Keywords: Arrhythmia marker; Bisphenol A; Canine heart; Low dose; QT prolongation; hERG channel.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
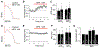
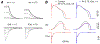
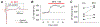
Comment in
-
Choice of experimental model determines translational impact: The link between bisphenol A and cardiotoxicity.Food Chem Toxicol. 2023 Apr;174:113667. doi: 10.1016/j.fct.2023.113667. Epub 2023 Feb 13. Food Chem Toxicol. 2023. PMID: 36791906 Free PMC article. No abstract available.
References
-
- Abi-Gerges N, Philp K, Pollard C, Wakefield I, Hammond TG, Valentin JP, 2004. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol 18, 139–151. - PubMed
-
- Arias-Loza PA, Jazbutyte V, Pelzer T, 2008. Genetic and pharmacologic strategies to determine the function of estrogen receptor alpha and estrogen receptor beta in cardiovascular system. Gend Med 5 Suppl A, S34–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

