The Effect of high temperature on the stability of basal insulin in a pen: a randomized controlled, crossover, equivalence trial
- PMID: 36585035
- PMCID: PMC9809263
- DOI: 10.1136/bmjdrc-2022-003105
The Effect of high temperature on the stability of basal insulin in a pen: a randomized controlled, crossover, equivalence trial
Abstract
Introduction: Insulin is an essential medicine in the management of diabetes. When stored at high temperatures(HTs), its efficacy could rapidly decline. Therefore, appropriate storage of in-use insulin is necessary to achieve its maximum therapeutic effects. However, the ambient temperature in tropical countries is normally relatively high. This study aimed to compare the efficacies of basal insulin in a pen previously kept at 37°C for 21 days and basal insulin in a refrigerated pen (2°C-8°C). Continuous glucose monitoring (CGM) was used to evaluate daily mean glucose levels (MGLs).
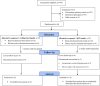
Research design and methods: This randomized controlled, crossover, equivalence trial recruited adults with type 2 diabetes mellitus and glycated hemoglobin levels <8% who had used insulin glargine for >3 months. Subjects were randomized for sequential use of refrigerated basal insulin followed by basal insulin kept at HT, with a 2-week washout between phases. The HT insulin pens were stored in a 37°C incubator for 21 days before use, while the refrigerated insulin pens were stored at 2°C-8°C. Study patients received 7-day CGM. The primary outcome was the difference in the groups' MGLs. The secondary outcome parameters were glucose variability represented by the standard deviation (SD), mean amplitude of glycemic excursion (MAGE), and percentage of time in range (TIR). The remaining quantity of insulin was evaluated by ultrahigh-performance liquid chromatography (UHPLC) assay.
Results: Forty patients completed the study. The MGLwas 158.7±30.5 mg/dL and 157.0±40.9 mg/dL in the HT and refrigerated insulin pen groups, respectively (p=0.72). The groups had no significant differences in MAGE7day, SD, percentage of TIR, carryover period, or treatment effects (all p>0.05). There was also no significant difference in the remaining quantity of insulin evaluated by UHPLC (p=0.97).
Conclusions: HT basal insulin pens retain their potency and have biological activity comparable to that of refrigerated pens.Trial registration number TCTR20210611002.
Keywords: Continuous Glucose Monitoring; Diabetes Mellitus, Type 2; Insulin; Patient Education as Topic.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- International Diabetes Federation . IDF diabetes atlas. 10th edn. Brussels, Belgium: International Diabetes Federation, 2021.
-
- Weiss M. Design of ultra-stable insulin analogues for the developing world. Journal of Health Specialties 2013;1:59–70. 10.4103/1658-600X.114683 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
