Roles of oral microbiota and oral-gut microbial transmission in hypertension
- PMID: 36585105
- PMCID: PMC9811375
- DOI: 10.1016/j.jare.2022.03.007
Roles of oral microbiota and oral-gut microbial transmission in hypertension
Abstract
Introduction: Considerable evidence has linked periodontitis (PD) to hypertension (HTN), but the nature behind this connection is unclear. Dysbiosis of oral microbiota leading to PD is known to aggravate different systematic diseases, but the alteration of oral microbiota in HTN and their impacts on blood pressure (BP) remains to be discovered.
Objectives: To characterize the alterations of oral and gut microbiota and their roles in HTN.
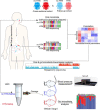
Methods: We performed a cross-sectional (95 HTN participants and 39 controls) and a 6-month follow-up study (52 HTN participants and 26 controls) to analyze the roles of oral and gut microbiota in HTN. Saliva, subgingival plaques, and feces were collected for 16S rRNA gene sequencing or metagenomic analysis. C57BL/6J mice were pretreated with antibiotics to deplete gut microbiota, and then transplanted with human saliva by gavage to test the impacts of abnormal oral-gut microbial transmission on HTN.
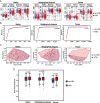
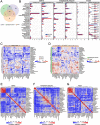
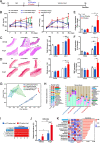
Results: BP in participants with PD was higher than no PD in both cross-sectional and follow-up cohort. Relative abundances of 14 salivary genera, 15 subgingival genera and 10 gut genera significantly altered in HTN and those of 7 salivary genera, 12 subgingival genera and 6 gut genera significantly correlated with BP. Sixteen species under 5 genera were identified as oral-gut transmitters, illustrating the presence of oral-gut microbial transmission in HTN. Veillonella was a frequent oral-gut transmitter stably enriched in HTN participants of both cross-sectional and follow-up cohorts. Saliva from HTN participants increased BP in hypertensive mice. Human saliva-derived Veillonella successfully colonized in mouse gut, more abundantly under HTN condition.
Conclusions: PD and oral microbiota are strongly associated with HTN, likely through oral-gut transmission of microbes. Ectopic colonization of saliva-derived Veillonella in the gut may aggravate HTN. Therefore, precise manipulations of oral microbiota and/or oral-gut microbial transmission may be useful strategies for better prevention and treatment of HTN.
Keywords: Blood pressure; Gut microbiota; Hypertension; Oral microbiota; Oral-gut axis; Veillonella.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-104. - PubMed
-
- Tsioufis C., Kasiakogias A., Thomopoulos C., Stefanadis C. Periodontitis and blood pressure: the concept of dental hypertension. Atherosclerosis. 2011;219(1):1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

