Cohort Profile:The Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE)
- PMID: 36585137
- PMCID: PMC9809224
- DOI: 10.1136/bmjopen-2022-069065
Cohort Profile:The Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE)
Abstract
Purpose: The ENFORCE cohort is a national Danish prospective cohort of adults who received a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine as part of the Danish National SARS-CoV-2 vaccination programme. It was designed to investigate the long-term effectiveness, safety and durability of SARS-CoV-2 vaccines used in Denmark.
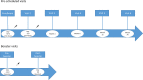
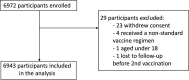
Participants: A total of 6943 adults scheduled to receive a SARS-CoV-2 vaccine in the Danish COVID-19 vaccination programme were enrolled in the study prior to their first vaccination. Participants will be followed for a total of 2 years with five predetermined follow-up visits and additional visits in relation to any booster vaccination. Serology measurements are performed after each study visit. T-cell immunity is evaluated at each study visit for a subgroup of 699 participants. Safety information is collected from participants at visits following each vaccination. Data on hospital admissions, diagnoses, deaths and SARS-CoV-2 PCR results are collected from national registries throughout the study period. The median age of participants was 64 years (IQR 53-75), 56.6% were women and 23% were individuals with an increased risk of a serious course of COVID-19. A total of 340 (4.9%) participants tested positive for SARS-CoV-2 spike IgG at baseline.
Findings to date: Results have been published on risk factors for humoral hyporesponsiveness and non-durable response to SARS-CoV-2 vaccination, the risk of breakthrough infections at different levels of SARS-CoV-2 spike IgG by viral variant and on the antibody neutralising capacity against different SARS-CoV-2 variants following primary and booster vaccinations.
Future plans: The ENFORCE cohort will continuously generate studies investigating immunological response, effectiveness, safety and durability of the SARS-CoV-2 vaccines.
Trial registration number: NCT04760132.
Keywords: COVID-19; IMMUNOLOGY; Infection control.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NBS declares to have served as investigator on studies sponsored by Pfizer, Gilead, Bavarian and Sanofi Pasteur. HN declares receiving a grant from Novo Nordisk Foundation and to have been on advisory boards for MSD and GSK. TB declares receipt of unrestricted research or travel grants from GSK, Pfizer, Gilead Sciences, MSD; and being principal investigator on trials conducted by Boehringer Ingelheim, Roche, Novartis, Kancera, Pfizer, MSD and Gilead; Board member on Pentabase, and advisory board member for MSD, Gilead, Pfizer, GSK, Janssen and AstraZeneca; consulting fees from GSK and Pfizer; receiving donation of study drug from Eli Lilly; and receiving honorarium for lectures from GSK, Pfizer, Gilead Sciences, Boehringer Ingelheim, AbbVie and AstraZeneca.
Figures


References
-
- Logunov DY, Dolzhikova IV, Zubkova OV, et al. . Safety and immunogenicity of an Rad26 and RAD5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020;396:887–97. 10.1016/S0140-6736(20)31866-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
