Introducing ROTAVAC® to the occupied Palestinian Territories: Impact on diarrhea incidence, rotavirus prevalence and genotype composition
- PMID: 36585280
- PMCID: PMC9880560
- DOI: 10.1016/j.vaccine.2022.12.046
Introducing ROTAVAC® to the occupied Palestinian Territories: Impact on diarrhea incidence, rotavirus prevalence and genotype composition
Abstract
Background: Rotavirus infection remains an important cause of morbidity and mortality in children. The introduction of vaccination programs in more than 100 countries has contributed to a decrease in hospitalizations and mortality. This study investigates the epidemiological impact of the rotavirus vaccine ROTAVAC® in the Palestinian Territories, the first country to switch from ROTARIX® to this new vaccine.
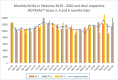
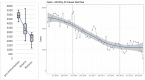
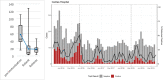
Methods: Clinical surveillance data was collected fromchildren younger than 5attendingoutpatient clinics throughout Gaza withdiarrhea between 2015 and 2020. The incidence of all-cause diarrhea was assessed using an interrupted time-series approach. Rotavirus prevalence was determined at the Caritas Baby Hospital in the West Bank usingELISA on stool specimen of children younger than 5with diarrhea. Genotyping was performed on 325 randomly selected rotavirus-positive samples from January 2015 through December 2020 using multiplex PCR analysis.
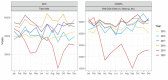
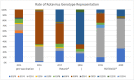
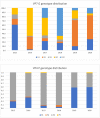
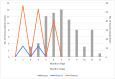
Results: Average monthly diarrhea casesdropped by 16.7% annually fromintroduction of rotavirus vaccination in May 2016 to the beginning of the SARS-CoV-2 epidemic in March 2020 for a total of 53%. Case count declines were maintained afterthe switchto ROTAVAC® in October 2018. Rotavirus positivity in stool samples declined by 67.1% over the same period without change followingthe switch to ROTAVAC®. The distribution of predominant genotypes in rotavirus-positive stool samples changed from a pre-vaccination G1P [8] to G9P[8] and G12P[8] during the ROTARIX® period and G2P[4] after the introduction of ROTAVAC®.
Conclusion: ROTAVAC® has shown epidemiological impact on par with ROTARIX® after its introduction to the national immunization schedule in the Palestinian Territories. A molecular genotype shift from a pre-vaccination predominance of G1P[8] to a current predominance of G2P[4] requires more long-term surveillance.
Keywords: Genotyping; Rotavirus; Vaccine impact.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [Wolfgang Rennert MD reports financial support was provided by Bill & Melinda Gates Foundation].
Figures
Similar articles
-
Epidemiology and genetic diversity of group A rotavirus in acute diarrhea patients in pre-vaccination era in Himachal Pradesh, India.Vaccine. 2019 Aug 23;37(36):5350-5356. doi: 10.1016/j.vaccine.2019.07.037. Epub 2019 Jul 19. Vaccine. 2019. PMID: 31331769
-
Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar.Vaccine. 2018 Nov 12;36(47):7198-7204. doi: 10.1016/j.vaccine.2017.08.091. Epub 2017 Sep 25. Vaccine. 2018. PMID: 28958809 Free PMC article.
-
Challenges and lessons learned during the switching of rotavirus vaccine from Rotarix to Rotavac in Zambia.Vaccine. 2025 May 10;55:127012. doi: 10.1016/j.vaccine.2025.127012. Epub 2025 Mar 18. Vaccine. 2025. PMID: 40107130
-
Efficacy of rotavirus vaccines in Indonesia: A review of genotype distribution and impact.Narra J. 2025 Apr;5(1):e1681. doi: 10.52225/narra.v5i1.1681. Epub 2025 Feb 10. Narra J. 2025. PMID: 40352187 Free PMC article. Review.
-
Dynamics of G2P[4] strain evolution and rotavirus vaccination: A review of evidence for Rotarix.Vaccine. 2020 Jul 31;38(35):5591-5600. doi: 10.1016/j.vaccine.2020.06.059. Epub 2020 Jul 7. Vaccine. 2020. PMID: 32651115 Review.
Cited by
-
Genetic diversity of G9, G3, G8 and G1 rotavirus group A strains circulating among children with acute gastroenteritis in Vietnam from 2016 to 2021.Infect Genet Evol. 2024 Mar;118:105566. doi: 10.1016/j.meegid.2024.105566. Epub 2024 Feb 3. Infect Genet Evol. 2024. PMID: 38316245 Free PMC article.
-
Antimicrobial resistance in a protracted war setting: a review of the literature from Palestine.mSystems. 2025 Jun 17;10(6):e0167924. doi: 10.1128/msystems.01679-24. Epub 2025 May 21. mSystems. 2025. PMID: 40397600 Free PMC article. Review.
References
-
- Troeger C., Blacker B.F., Khalil I.A., et al. Estimates of global, regional, and national morbidity, mortality, and aetiologias of diarrhea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
-
- Aliabadi N., Antoni S., Mwenda J.M., et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7:e893–e903. doi: 10.1016/S2214-109X(19)30207-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

